SGGP
The UK's Medicines and Healthcare products Regulatory Agency has approved Casgevy - the world's first CRISPR gene-editing treatment - to treat sickle cell disease (pictured), opening up hope of a cure for thousands of patients.
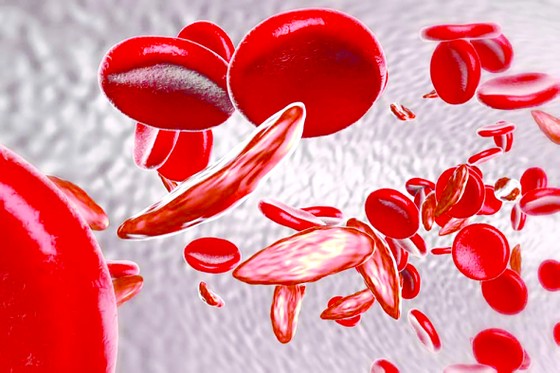 |
The agency has approved Casgevy for patients aged 12 and older with sickle cell disease and thalassemia. Casgevy works by targeting the faulty genes in a patient’s bone marrow stem cells, which help the body produce normal-functioning hemoglobin. The first step is chemotherapy. Doctors then take stem cells from the bone marrow and genetically modify them in a lab. The cells are then infused back into the patient for long-term treatment.
Sickle cell disease and thalassemia result from a defect in the gene that carries hemoglobin, the protein in red blood cells that carries oxygen. In people with sickle cell disease, the gene mutation causes the cells to take on a sickle shape, blocking blood flow and causing severe pain, organ damage, strokes, and other problems. The disease is especially common in people of African or Caribbean descent.
Source



























































































Comment (0)