The Ministry of Health is seeking opinions on two draft circulars related to the list of pharmaceutical drugs, traditional medicine... in health insurance examination and treatment.

Deputy Minister of Health Tran Van Thuan speaks at the workshop to collect opinions on the development of a circular on the list and payment rate of drugs in health insurance examination and treatment - Photo: Ministry of Health
Health insurance drugs in Vietnam have good coverage
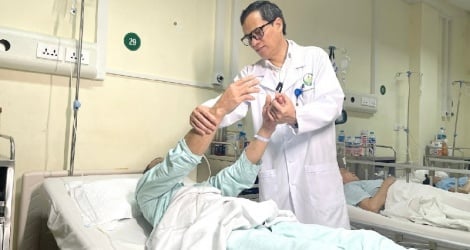
On October 25, the Ministry of Health held a workshop to collect opinions on the development of two draft circulars related to the list, rates and payment conditions for pharmaceutical drugs, biological products, traditional medicine, etc. in medical examination and treatment under health insurance.
Speaking at the workshop, Deputy Minister of Health Tran Van Thuan emphasized that medicine always accounts for a large proportion of health insurance examination and treatment costs.
Although this expenditure ratio has gradually decreased over the years, drug costs remain high, specifically in 2020 it was 40.42 trillion VND (34.75%), in 2021 it was 34.48 trillion VND (34.86%) and in 2022 it was 40.57 trillion VND (33.41%).
Currently, payment of drug costs for health insurance participants is in accordance with Circular 20/2022/TT-BYT with 1,037 active ingredients/pharmaceutical drugs, biological products and 59 radioactive drugs and markers in the list of health insurance participants.
Deputy Minister Thuan also said that the list of health insurance drugs in Vietnam has better coverage than that of regional countries such as Thailand and Singapore.
"Currently, there are some shortcomings in Circular 22 that need to be adjusted, especially related to drug payment for remote medical examination and treatment - an area that has been regulated in the 2023 Law on Medical Examination and Treatment, but has not been reflected in the list of health insurance drugs," said Mr. Thuan.
4 new points in the draft circular
At the workshop, Ms. Tran Thi Trang, Director of the Department of Health Insurance (Ministry of Health), shared 4 notable new points in this revision.
Firstly, the draft will update new drugs with reasonable costs and high treatment effectiveness, to meet the needs of diagnosis and treatment at all levels from central to primary health facilities.
Second, the drafting committee will review the entire current list, removing drugs that have warnings related to treatment or are no longer suitable in terms of cost and effectiveness. This will help health insurance participants use drugs more effectively and reasonably.
Third, the new circular will adjust the principles and criteria for including or removing drugs from the health insurance list, ensuring quick, objective and scientific updates, while still balancing the health insurance fund. Lower-level health facilities will be given priority access to the most effective treatment drugs, suitable to the professional capacity of each facility.
Finally, this draft circular also adjusts the payment principles to ensure flexibility in accordance with the professional regulations of the 2023 Law on Medical Examination and Treatment. Accordingly, any medical examination and treatment facility with sufficient treatment capacity can pay for drugs according to the disease, regardless of the level.
Ms. Trang also said that updating the drug list will take place regularly, at least once a year, with special priority given to creating favorable conditions for commune health stations, helping lower levels expand the right to use drugs to meet the timely treatment needs of patients.
Experts also said that the development of the circular contributes to the rational, safe and effective use of drugs; meets treatment needs, is suitable to the disease structure, ensures the rights of health insurance participants and the payment capacity of the health insurance fund.
Source: https://tuoitre.vn/se-ra-soat-cap-nhat-thuoc-moi-trong-danh-muc-thuoc-bao-hiem-y-te-20241025152706616.htm
























































































Comment (0)