On February 24, the Drug Administration of Vietnam (Ministry of Health) announced the suspension of circulation and nationwide recall of Peeling acne serum products manufactured by Bitechpharm Biotechnology Joint Stock Company and E-Cosmetic Face wash gel products manufactured by LAP Vietnam Pharmaceutical Company Limited and distributed to the market by Long Phung Group Company Limited. These companies are all headquartered in Ho Chi Minh City.
Reason for recall: product uses ingredients not listed in cosmetic product declaration file.
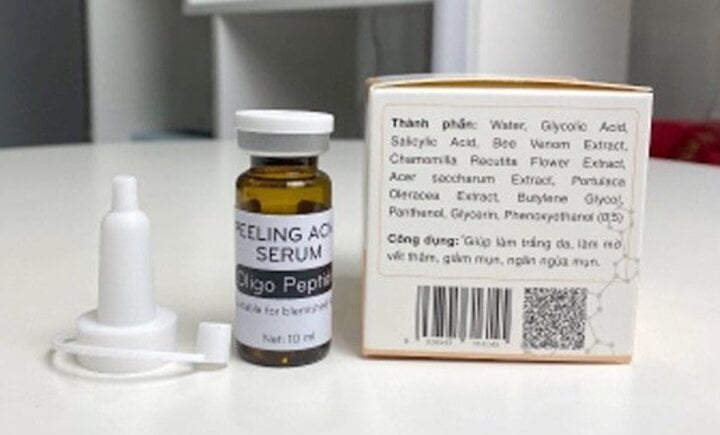
Peeling acne serum product is manufactured by Bitechpharm company.
The Drug Administration of Vietnam requests the Department of Health of provinces and centrally-run cities to notify cosmetic businesses and users in the area to stop selling the Peeling acne serum and E-Cosmetic Face wash gel products and return them to the product suppliers.
Bitechpharm Biotechnology Joint Stock Company, LAP Vietnam Pharmaceutical Company Limited, Long Phung Group Company Limited, send recall notices to distributors and users of the above product batch.
At the same time, receive returned products from businesses, recall and destroy all cosmetic batches that do not meet regulations. Send a report to the Department of Drug Administration before March 31.
The Department requested the Ho Chi Minh City Department of Health to withdraw the Cosmetic Product Declaration receipt number of the two cosmetic batches above. Supervise companies to recall and destroy the Peeling acne and E-Cosmetic Face wash gel product batches and report before April 15.
Source


























































































Comment (0)