On November 25, information from the Can Tho City Department of Health said that it had sent an official dispatch requesting Can Tho Dermatology Hospital to stop using technical procedures using the needle rolling and micro-needle methods at the hospital.

Can Tho Dermatology Hospital - Photo: T.LUY
Previously, public opinion reflected that Can Tho Dermatology Hospital used 4 types of topical cosmetics on prescription samples, and used Hydro Injector II to inject nutrients into the patient's face for a long time to treat melasma.
Here are four cosmetics: Goodndoc Hydra B5 Serum; Goodndoc Vitamin C-16.5 Daily Whitening Serum; Beauty Skin Vita C; Beauty Skin EGF Moisture.
According to the feedback, these products are issued by the Department of Drug Administration (Ministry of Health); in the instructions for use, these products are in the form of "cream, emulsion, milk, gel or oil for use on the skin (face, feet, hands, etc.)".
After receiving public feedback, Can Tho Department of Health requested a report and received feedback from the hospital on the process of using cosmetic products with multi-needle nutrient injection machines.
The Department of Health has issued a document to rectify the management and use of cosmetic products at hospitals, requiring: "The Director of Can Tho Dermatology Hospital is responsible for directing the management and use of drugs and cosmetic products in accordance with current regulations. The use of cosmetic products must comply with the manufacturer's instructions.
Regarding professional technical procedures, they must comply with the instructions of the Ministry of Health. In case the hospital applies new methods and techniques in medical examination and treatment, it must carry out procedures for submission to competent authorities for approval according to the current Law on Medical Examination and Treatment.
However, on November 22, the Department of Health Inspectorate continued to receive a complaint about the use of the above cosmetics at the Dermatology Hospital. Therefore, the Department requested to immediately stop using this technical process; requested the professional departments and the Department of Health Inspectorate to clarify the professional technical process and cosmetic drugs used at the hospital. When there is a conclusion, the Ministry of Health will be informed and the public will be clearly informed about this issue.
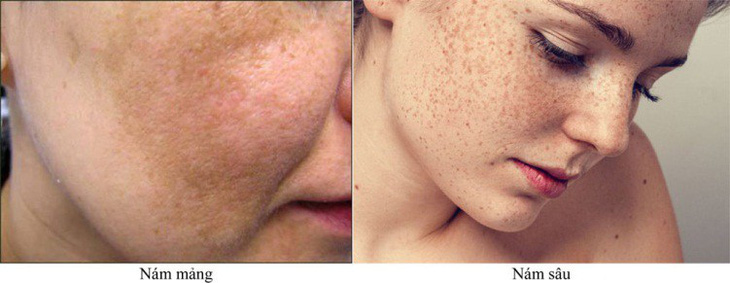 Why skin gets melasma and how to treat it
Why skin gets melasma and how to treat itSource: https://tuoitre.vn/dung-my-pham-thoa-da-tiem-vao-mat-benh-nhan-benh-vien-da-lieu-can-tho-bi-thanh-tra-20241125184320364.htm




![[Photo] Prime Minister Pham Minh Chinh chairs the regular Government meeting in April 2025](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/6/48eb0c5318914cc49ff858e81c924e65)























![[Photo] Overseas Vietnamese in France review history through special supplement commemorating 50 years of national reunification](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/6/e92838061d514fceb6edc82f751aafef)








































































Comment (0)