Ho Chi Minh City authorities announced the results of administrative sanctions against establishments violating the fields of pharmaceuticals, cosmetics and medical equipment.
Ho Chi Minh City authorities announced the results of administrative sanctions against establishments violating the fields of pharmaceuticals, cosmetics and medical equipment.
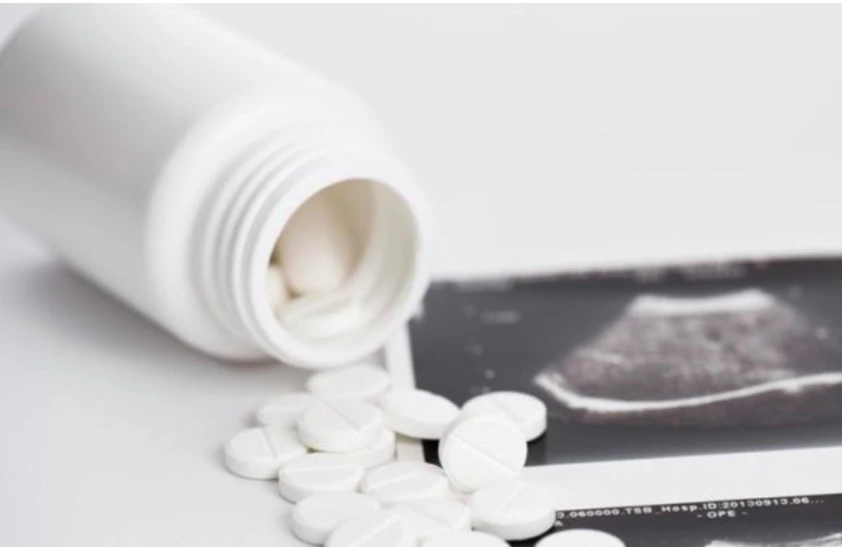
During this period, Francia Beauty Company Limited was heavily fined for violating the production and trading of underarm whitening cream and deodorant products containing illegal salicylic acid.
 |
| Francia Beauty Company Limited has been heavily fined for violating regulations in the production and trading of underarm whitening and deodorant creams containing illegal salicylic acid. |
Specifically, the company's Cléo underarm whitening and deodorant cream (1 tube of 35g) contained salicylic acid, an ingredient not included in the approved formula. The company violated regulations on cosmetics management in Vietnam.
In addition, the company also engaged in false advertising, marketing and product labeling practices, misleading consumers into believing that the product acted as a medicine.
For these violations, Francia Beauty Company Limited was fined 140 million VND. In addition, the Company was also required to recall and destroy the violating products, and return the cosmetic product declaration form issued by the Long An Department of Health on December 23, 2021.
In addition, the company also suspended nationwide circulation of the Cléo underarm whitening and deodorant cream batch (batch number: 120124, manufactured on January 12, 2024), after the product sample tested did not meet quality standards.
The Drug Administration of Vietnam (Ministry of Health) has decided to recall this product due to the discovery that it contains Salicylic Acid, an ingredient that is not allowed to be present in this cosmetic product.
In this round of fines, a number of other establishments were also fined. Specifically, Saigon Medical Services Company Limited (District 5, Ho Chi Minh City) was fined 230 million VND for borrowing a certificate of eligibility to do pharmaceutical business and trading in goods of unknown origin. Le Khang 2 Pharmacy was also fined 4 million VND for professional violations.
The Ministry of Health has requested the Ho Chi Minh City and Long An Departments of Health to monitor the recall of violating products and continue to inspect cosmetic production and trading establishments, and handle violations according to regulations.
To manage cosmetics, the Drug Administration has also sent documents to the Departments of Health and establishments producing, publishing, and importing cosmetics, requesting them to strengthen the management of cosmetics on the market.
Through inspection, the Department discovered that many businesses could not be contacted through the information on the Declaration Form or on the company's website. Some businesses changed their address and representative without notifying the authorities, causing difficulties in inspection and handling.
The Drug Administration of Vietnam requires the Departments of Health to coordinate with Steering Committee 389 and the Market Management Department to inspect the production and trading of cosmetics on social networking platforms and e-commerce platforms, in order to detect and handle fake cosmetic products, products of unknown origin or those with false advertising about features and uses.
To protect consumer rights and ensure public health safety, the Drug Administration of Vietnam requires strict handling of organizations and individuals who violate regulations in the production and trading of cosmetics.
Cosmetics suspected of being counterfeit, of unknown origin, or of substandard quality will be recalled and destroyed. Cosmetic manufacturing and trading establishments must fully comply with regulations, maintain product information records, and present them upon request from inspection agencies.
The Drug Administration of Vietnam also requires cosmetic manufacturing, importing and declaring establishments to comply with the regulations in Circular No. 06/2011/TT-BYT of the Ministry of Health, including declaring product declaration information accurately and honestly.
Each facility must keep a Product Information File (PIF) at its headquarters and provide it upon request from the authorities. Only when the product has been granted a Declaration receipt number, will the business be allowed to put cosmetics on the market.
The Drug Administration of Vietnam affirmed that it will continue to conduct post-market inspections to ensure that cosmetics circulating on the market fully meet requirements on safety, quality and effectiveness.
Source: https://baodautu.vn/bi-phat-nang-vi-ban-my-pham-chua-acid-salicylic-trai-phep-d256557.html






![[Photo] General Secretary To Lam and Prime Minister Pham Minh Chinh attend the first Congress of the National Data Association](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/3/22/5d9be594d4824ccba3ddff5886db2a9e)













![[Photo] Overview of the Workshop "Removing policy shortcomings to promote the role of the private economy in the Vietnamese economy"](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/3/21/d1c58c1df227467b8b33d9230d4a7342)






















































Comment (0)