In December 2024, the American scientific journal Science honored lenacapavir - a new generation of injectable drugs used to treat HIV-infected patients as "Breakthrough of the Year". A large-scale study showed that the drug is up to 100% effective in preventing the disease of the century.
Lenacapavir, sold under the brand name Sunlenca, is a new class of antiviral drugs developed by the biopharmaceutical company Gilead Sciences. It works by blocking the formation of the capsid, the protein shell that surrounds the genetic material of the HIV virus and is responsible for protecting the virus's genome.
The US Food and Drug Administration (FDA) has approved lenacapavir to treat patients with multidrug-resistant HIV since 2022. A large-scale study conducted by Gilead Sciences on young women and adolescent girls in Africa in June showed that the drug is up to 100% effective in preventing the disease of the century.
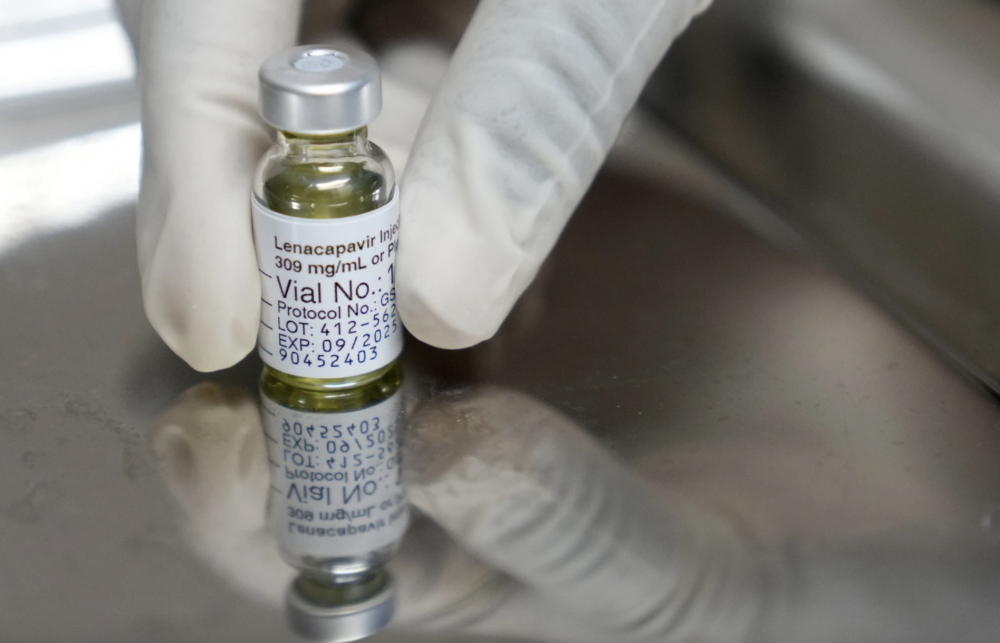
Large-scale clinical trials have shown that lenacapavir is almost completely effective in preventing HIV infection. A study of more than 5,000 young African women found no cases of HIV infection after lenacapavir injection, a 100% effectiveness. Another trial of more than 2,000 people of different genders also recorded a prevention effectiveness of up to 99.9%.
Unlike current antiviral drugs that target viral enzymes, lenacapavir inhibits the HIV envelope protein (capsid). This protein forms a protective shell around the virus's genetic material, allowing it to enter human cells. Lenacapavir binds to the capsid, stiffening the shell, preventing the virus from entering cells and replicating.
This new mechanism of action not only provides high preventive efficacy but also opens up prospects for developing similar drugs to treat other viral diseases.
Many HIV/AIDS researchers hope that the drug developed by US biopharmaceutical company Gilead Sciences will have a dramatic effect in reducing global infection rates when used as pre-exposure prophylaxis (PrEP).
The goal is to start distributing lenacapavir in late 2025 or early 2026, said Hui Yang, director of supply operations at the Global Fund to Fight AIDS, Tuberculosis and Malaria. But there are still many steps to complete, including getting the injection approved by regulatory agencies such as the US Food and Drug Administration (FDA) and the World Health Organization (WHO), Yang said.
“We don’t want low- and middle-income countries to wait and be left behind,” Ms. Yang stressed, pointing to decades of inequality in the fight against HIV.
According to Intellectual Property
Source: https://doanhnghiepvn.vn/cong-nghe/thuoc-dieu-tri-hiv-duoc-vinh-danh-dot-pha-cua-nam-2024/20241230103024549



![[Photo] Closing of the 11th Conference of the 13th Central Committee of the Communist Party of Vietnam](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/12/114b57fe6e9b4814a5ddfacf6dfe5b7f)
![[Photo] Overcoming all difficulties, speeding up construction progress of Hoa Binh Hydropower Plant Expansion Project](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/12/bff04b551e98484c84d74c8faa3526e0)























































































Comment (0)