After receiving an official dispatch from the Ho Chi Minh City Department of Health regarding support for the supply of drugs to treat hand, foot and mouth disease (Immunoglobulin, Phenobarbital) when the situation becomes complicated, the Department of Drug Administration, Ministry of Health has issued an official dispatch informing about the supply of drugs to support the treatment of severe hand, foot and mouth disease (Immunoglobulin, Phenobarbital).
Regarding drugs containing Immunoglobulin, according to the Drug Administration of Vietnam, there are currently 13 drugs containing Immunoglobulin that have been granted valid circulation registration certificates in Vietnam. According to reports from import establishments, there are currently a number of Immunoglobulin drugs in stock and plans to import them to Vietnam.
Human normal immunoglobulin 100mg/ml imported by Zuellig Pharma Vietnam Company has 2,344 boxes of 250ml and 215 boxes of 50ml left. It is expected that in mid-August 2023, the manufacturer will continue to supply Vietnam with 2,000 boxes of 250ml.
Regarding the 5% Immunoglobulin medicine imported by Duy Anh Pharmaceutical Trading Company Limited, Cho Ray Hospital currently has 300 bottles left. It is expected that by the end of July 2023, the manufacturer will supply Vietnam with 5,000 - 6,000 bottles.
Regarding Phenobarbital, there is currently one type of Phenobarbital manufactured by Danapha Pharmaceutical Joint Stock Company that has been granted a valid circulation registration certificate in Vietnam.
 |
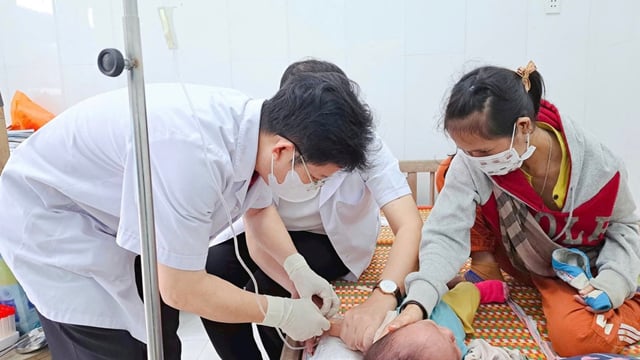
Examination of children with hand, foot and mouth disease. Photo: suckhoedoisong.vn |
The Drug Administration of Vietnam has granted permission to Central Pharmaceutical Joint Stock Company CPC1 to import Barbiturate, a drug that has not been registered for circulation in Vietnam, to meet special treatment needs.
According to the company's report, 21,000 vials of medicine (Phenobarbital 200mg/ml) will arrive in Vietnam in early July 2023.
To ensure adequate supply of drugs for treatment, the Drug Administration of Vietnam requests the Ho Chi Minh City Department of Health to develop and implement a plan for drug storage, procurement, and receipt in accordance with reality to ensure adequate supply of drugs and ensure medical examination and treatment.
At the same time, the Department urgently directed medical examination and treatment facilities in the area that need to use drugs promptly, proactively contacting import facilities to plan, order, purchase and reserve drugs in accordance with regulations. This is one of the important factors to ensure drug supply.
VNA
Source



![[Photo] Prime Minister Pham Minh Chinh chairs the Government's special meeting on law-making in April](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/13/8b2071d47adc4c22ac3a9534d12ddc17)




























![[Photo] Closing of the 11th Conference of the 13th Central Committee of the Communist Party of Vietnam](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/12/114b57fe6e9b4814a5ddfacf6dfe5b7f)


























































Comment (0)