 |
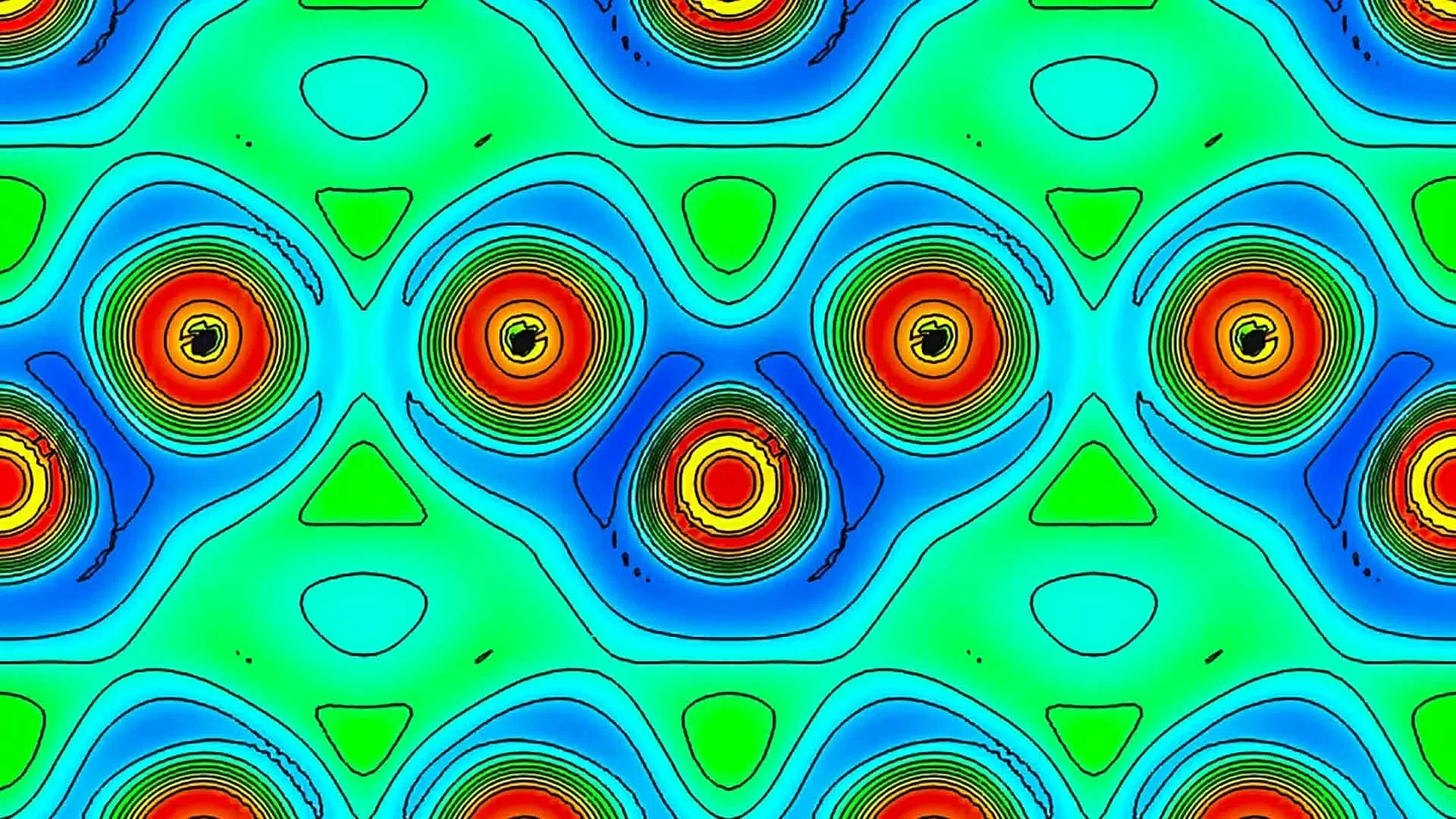
| At high temperature and pressure, helium atoms (red circles) move into the spaces between iron atoms (red circles with black centers) in a piece of iron metal on the electron localization function map. (Source: National Central University) |
Known as one of the most “recalcitrant” elements in the universe, helium is also the least reactive on the periodic table. Like other noble gases, helium does not easily gain or lose electrons, so it does not usually form chemical compounds. However, under extremely high pressures, helium can interact with a number of other elements, including nitrogen, sodium, and iron.
To create the new compound, University of Tokyo physicist Kei Hirose and colleagues compressed iron and helium together in a diamond anvil chamber — a high-pressure device that subjects the elements to pressures greater than 50,000 atm at temperatures above 1,000 degrees Celsius. This compression process creates crystals containing both iron and helium, which have a larger volume than pure iron crystals at the same pressure.
Scientists believe this increase is due to helium ions accumulating in interstitial spaces, tiny gaps between iron atoms in the crystal.
Although helium atoms are relatively unreactive, unable to form direct chemical bonds with iron even under extreme conditions, the compound that “packs” helium atoms into crystalline iron offers a “clue” that helps explain observations of helium in the Earth’s core. Most of the Earth’s helium atoms have two neutrons and are formed from the radioactive decay of elements such as uranium. However, some oceanic volcanic eruptions release helium atoms with just one neutron.
These atoms were first created shortly after the Big Bang. The Earth absorbed this “primordial” helium when the planet formed. The release of helium atoms from magma suggests that the Earth once had a primordial helium reserve, and the new compound suggests that the Earth’s iron-rich core likely contains the element.
In addition to their geophysical significance, these findings could have broader applications in noble gas chemistry. According to Maosheng Miao, a scientist at the University of California (USA), the formation of compounds containing helium and other metals could open up chemical insights that humans have never thought of.





![[Photo] Overcoming all difficulties, speeding up construction progress of Hoa Binh Hydropower Plant Expansion Project](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/12/bff04b551e98484c84d74c8faa3526e0)

![[Photo] Closing of the 11th Conference of the 13th Central Committee of the Communist Party of Vietnam](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/12/114b57fe6e9b4814a5ddfacf6dfe5b7f)













































































Comment (0)