This was emphasized by Dr. Nguyen Ngo Quang - Director of the Department of Science, Technology and Training, Ministry of Health at the conference "Ensuring the quality of research on the application of cell therapy and cell products in Vietnam" held on September 20 in Ho Chi Minh City.
Conference by The Department of Science, Technology and Training coordinated with the Ho Chi Minh City Department of Health to organize the conference. Attending and directing the conference were Dr. Nguyen Tri Thuc, Deputy Minister of Health.

Dr. Nguyen Tri Thuc, Deputy Minister of Health, gave a speech at the conference.
In recent years, scientific and technological activities and medical innovation have developed remarkably in both quantity and quality.
In particular, research and development of new products, techniques, and methods in general and research on the application of cells and cell-based products have initially introduced new protocols, techniques, and potential new products, providing physicians with more options to apply to medical examination and treatment, effectively serving the work of taking care of people's health.
The conference assessed the current status of research activities on cell applications and cell products in the period 2020 - 2024, and provided guidance on legal documents in the field of clinical trial research on new techniques, new methods, and cell application research.
From there, strengthen the management of clinical application research, ensure the quality of cells used in clinical trials; ensure scientific and ethical aspects in research; ensure the safety and effectiveness of research on cell applications and cell products.
The conference also assessed the current status and results of research activities on the application of cell therapy and cell products in the period 2020 - 2024; and unified the system of legal documents in the field of research on the application of cell therapy and cell products.
From there, strengthen the management of quality of research on cell applications and cell products in Vietnam.

Many delegates from agencies, organizations, professional associations and hospitals across the country attended the conference.
With the spirit of innovation and synchronization of legal corridor, development orientation, implementation, quality assurance, technology transfer/product commercialization.
In the coming time, research on the application of cells and cell products will develop in the right direction, comply with legal regulations, integrate with the region and the world, and bring more practical results to serve the work of caring for and protecting people's health.
Dr. Nguyen Ngo Quang - Director of the Department of Science, Technology and Training said: "The Ministry of Health also consults practical applications in regions and countries to incorporate into the legal content with the goal of creating conditions for the development of new medical research, new techniques, new technologies to serve people's health care, especially in the fields of biotechnology and regenerative medicine. In particular, the field of cell therapy and cell products is of great interest to the Ministry of Health.
In legal documents, especially the Law on Medical Examination and Treatment, a chapter has been included related to research and application of new methods and new techniques, creating a legal corridor with sufficient conditions to develop issues related to cell therapy and cell products.
The Ministry of Health has determined to create a legal corridor with sufficient conditions for units to research, apply and provide health care regimens and treatments for the people.
Dr. Quang also emphasized that units need to comply with regulations to ensure safety and absolute efficiency for people.
In addition, issues related to technical standards, improving the capacity and qualifications of human resources serving this field, standardizing processes and issues related to ensuring the quality of cell products before use on humans.
These are important requirements to help bring absolute effectiveness and safety when applying stem cell therapy in Vietnam.

Dr. Nguyen Ngo Quang reported at the conference on management work in the following years.
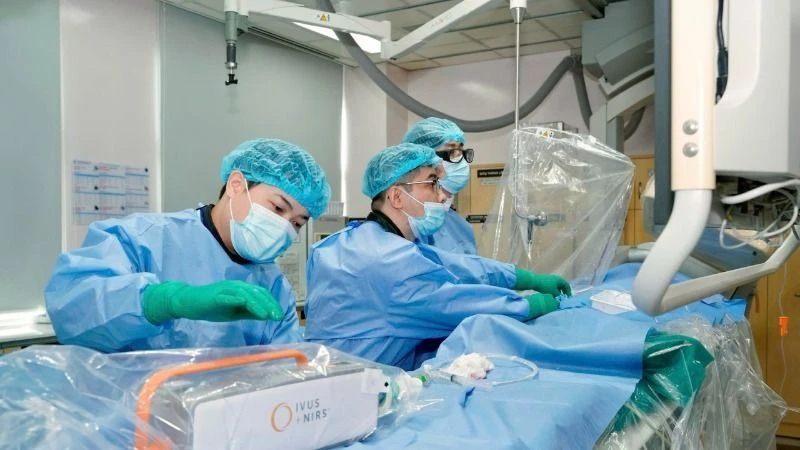
As one of the few pioneering hospitals investing in research on the latest therapies for applying stem cells in treatment and supporting disease treatment, the Stem Cell Center, Tam Anh General Hospital has been licensed to operate by the Ministry of Health since July 2019.
The center has invested in a modern, synchronous equipment system, using the most advanced techniques in biomedicine to help process, analyze, and accurately evaluate the quality of each cell sample; operating in a closed system right at the hospital from collection, reception, processing, preservation, storage, culture, production, application in disease treatment, research and training.
Dr. Tham Thi Thu Nga, Director of Tam Anh Stem Cell Center, said that the potential applications of cells and cell products are undeniable.
There is an increasing amount of research into stem cell therapy. The number of clinical trials is also increasing. Cell-based therapy is an advanced therapy, with many reports on its safety and effectiveness in treatment.
The future of cell therapy is very promising following the general trend of the world. Vietnam also has many studies applying stem cells in therapy.
However, cells and cell products are the most complex products among therapeutic drugs. Therefore, it requires time for research and close monitoring.

Research on stem cell applications in therapy is conducted at Tam Anh General Hospital.
According to Dr. Thu Nga, Tam Anh General Hospital system added that the Ministry of Health has licensed clinical trials using mesenchymal stem cells in the treatment of osteoarthritis.
This trial strictly followed the regulations of the Ministry of Health and the National Ethics Council to ensure the benefits of patients as well as the reliability of the study.

Dr. Tham Thi Thu Nga, Director of Tam Anh Stem Cell Center, spoke at the conference.
With the advantage of full and synchronous investment in infrastructure, equipment and well-trained human resources to develop applied research, Tam Anh General Hospital System is confident in meeting the requirements of the management agency.
From highly reliable research results, Tam Anh General Hospital system will make meaningful contributions to the development of applications in the cell field.
At the same time, work with management agencies to gradually remove difficulties in applying cell therapy in practice and contribute practical ideas to management agencies to help closely manage this therapy, helping people understand and correctly understand cell therapy.
Source: https://www.baogiaothong.vn/dam-bao-chat-luong-nghien-cuu-ung-dung-tri-lieu-te-bao-va-san-pham-tu-te-bao-o-viet-nam-19224092015094117.htm




![[Photo] Third meeting of the Organizing Subcommittee serving the 14th National Party Congress](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/2/3f342a185e714df58aad8c0fc08e4af2)
![[Photo] General Secretary To Lam receives Russian Ambassador to Vietnam](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/2/b486192404d54058b15165174ea36c4e)


























![[Photo] Relatives of victims of the earthquake in Myanmar were moved and grateful to the rescue team of the Vietnamese Ministry of National Defense.](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/2/aa6a37e9b59543dfb0ddc7f44162a7a7)














































![[Podcast] News April 2, 2025](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/2/eaa5bcbdb47a439bb7c43a417033535c)
![[Infographic] How does the State Bank of Region 12, including the Southeast provinces, operate?](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/2/91d2fdb1645c450a90c10104437b775b)
















Comment (0)