Expensive raw materials, complex manufacturing and a difficult-to-replicate model mean that Zolgensma, a drug to treat spinal muscular atrophy, is priced at $2.1 million (VND50 billion) per dose.
The once-in-a-lifetime drug treats spinal muscular atrophy (SMA), a genetic disorder that causes muscle wasting and weakness. Developed by AveXis, a subsidiary of US pharmaceutical giant Novartis, the drug was said to be the most expensive in the world at the time of its launch. Some insurance companies did not cover the drug because of its high price. Others had strict requirements to limit the number of patients who were eligible.
Seven Vietnamese children with spinal muscular atrophy have been provided free medicine by AveXis through a lottery program held every two weeks.
Breakthrough treatment effectiveness
According to experts, to understand why a dose of Zolgensma costs nearly 50 billion VND, it is necessary to learn about how and why this drug was created. Zolgensma belongs to the group of personalized drugs, targeting specific problems caused by a patient's genetic code. Thus, this method of treating the disease is much more effective than traditional methods, which are common and available to many patients.
Zolgensma is essentially a type of gene therapy. The drug replaces the faulty SMN1 gene in people with spinal muscular atrophy with a new, normally functioning copy.
But getting this healthy copy into cells isn’t easy. The new gene must be placed inside a viral vector, called adenovirus type 9 (AAV9). Scientists remove some of the virus’s DNA so it can’t cause disease in humans. Once introduced into the body via an intravenous infusion, the viral vector travels to cells, carrying the new, healthy gene to replace the faulty one.
The drug also contains the active ingredient onasemnogene abeparvovec, which helps restore genes and produce proteins for muscle movement and nerve function. After Zolgensma was introduced, about 80 children in the UK were saved from critical condition each year.
Manufacturer Novartis says Zolgensma is expensive because it “significantly changes the lives of families affected by a devastating disease.” Every year, more than 60,000 children worldwide are diagnosed with spinal muscular atrophy. Since its launch, Zolgensma has been approved in about 38 countries, with more than 1,000 children receiving the drug.
Novartis CEO Vas Narasimhan argues that gene therapies represent a medical breakthrough, offering the hope of curing deadly genetic diseases with just one dose.
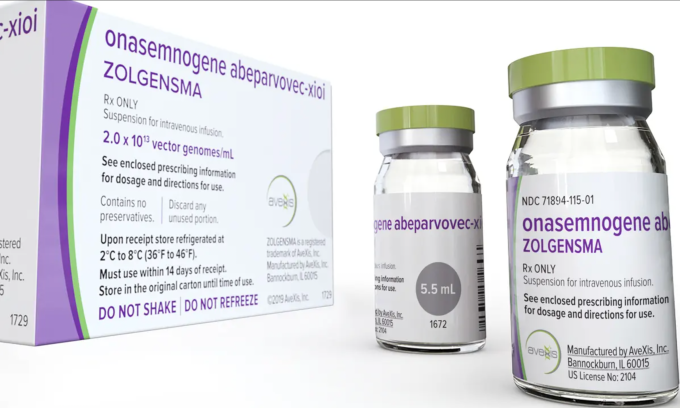
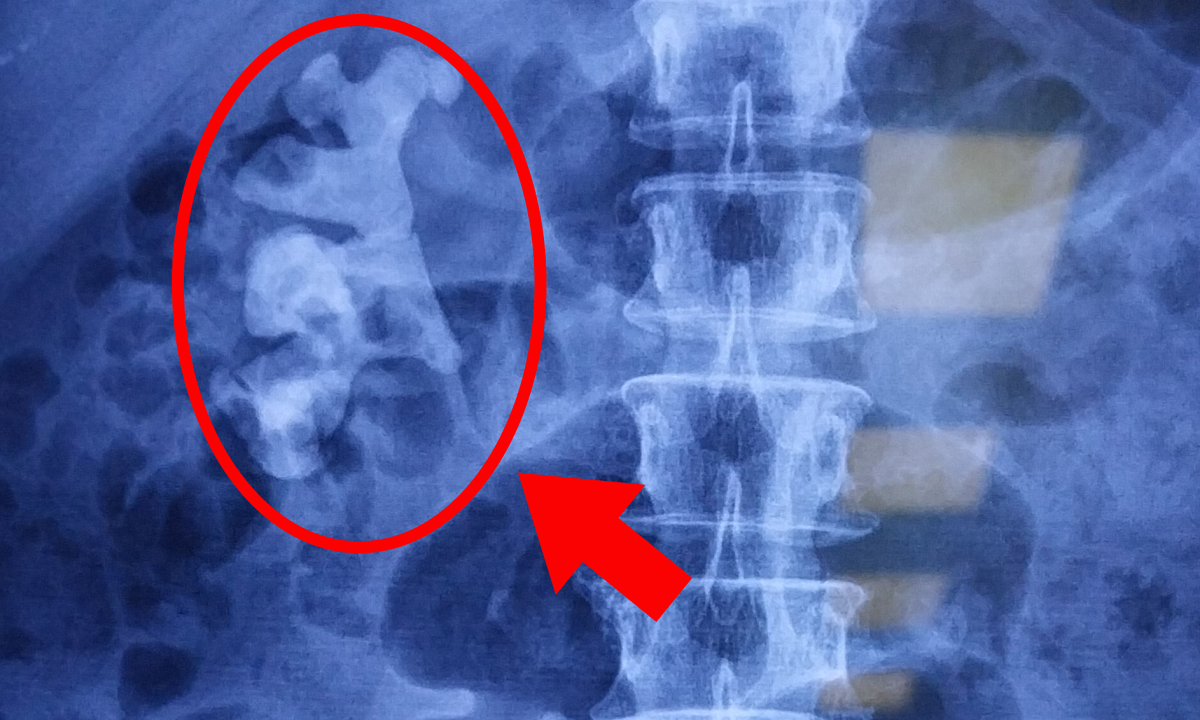
Zolgensma drug costs 2.1 million USD. Photo: Novartis
Difficult to replicate production model
While gene therapy is a promising treatment option for many diseases, the process of manufacturing the drugs is difficult. Unlike antibody treatments, which allow for the creation and expansion of cell lines, gene therapy is difficult to scale up and very expensive to produce.
The problem lies in the biology, because the original AAV9 vector is a virus that wants to kill cells. Once the DNA is removed and replaced, the virus is safe to introduce into human cells. However, the process to get to this stage is extremely expensive.
Companies are racing to develop and market new drugs. But they don’t spend much time optimizing their manufacturing processes. As a result, drugs are rushed to market at high costs.
“Right now, the world doesn’t have the capacity, the manufacturing processes to make drugs for rare or extremely rare diseases. For gene therapies, you have to buy very expensive raw materials. So the ability to scale up and operate this model is very limited,” explains Eric Hobbs, CEO of Berkeley Lights.
Zolgensma is currently a proprietary drug manufactured by Novartis, with no biosimilar version available - a drug that is similar in quality, safety, and clinical effectiveness to the original product.
Zolgensma is also considered a biologic drug, meaning it is made from living cells. Therefore, scientists cannot create exact copies of these drugs.
The alternative to Zolgensma is Spinraza, which is taken four times a year for life. The drug’s list price is $750,000 for the first year and $350,000 per year after that, or about $4 million over 10 years.
The nonprofit group Institute for Clinical Economics Review estimates that the fair price for new gene therapies is between $1.2 million and $2.1 million.
Thuc Linh (According to Forbes, Guardian, Healthline )
Source link



![[Photo] Overcoming all difficulties, speeding up construction progress of Hoa Binh Hydropower Plant Expansion Project](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/12/bff04b551e98484c84d74c8faa3526e0)



![[Photo] Closing of the 11th Conference of the 13th Central Committee of the Communist Party of Vietnam](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/12/114b57fe6e9b4814a5ddfacf6dfe5b7f)














































































Comment (0)