SGGPO
RSV is a highly contagious virus that causes pneumonia and respiratory infections. RSV enters the body through the eyes, nose or mouth.
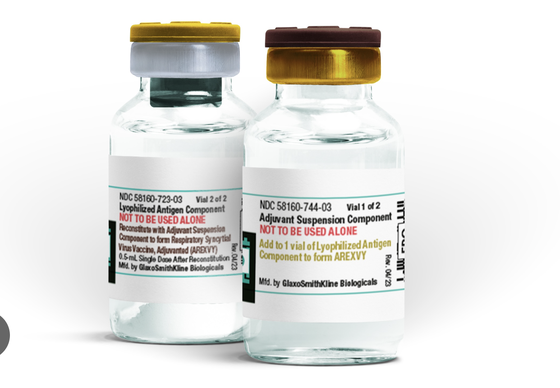 |
| GSK's Arexvy vaccine has been licensed by the EU for use in people over 60 years old. |
On June 7, British pharmaceutical company GlaxoSmithKline (GSK) said the European Commission (EC) has licensed the world's first vaccine against respiratory syncytial virus (RSV).
Specifically, GSK's Arexvy vaccine has been licensed for use in people over 60 years old.
In a statement, GSK CEO Tony Wood said the decision to license the Arexvy vaccine means eligible adults can be vaccinated against RSV for the first time.
RSV is a highly contagious virus that causes pneumonia and respiratory infections. RSV enters the body through the eyes, nose, or mouth. It is easily transmitted from person to person through infected respiratory secretions such as coughing, sneezing, or direct contact such as shaking hands. RSV usually causes mild symptoms, but can cause severe illness in infants, young children, and the elderly, as well as people with underlying medical conditions and weakened immune systems.
Previously, on May 3, US authorities made a similar decision, approving the Arexvy vaccine, becoming the first country in the world to approve an RSV vaccine.
On May 31, Pfizer announced that the US Food and Drug Administration (FDA) had approved the Abrysvo RSV vaccine produced by the company. Accordingly, this is the second RSV vaccine approved by the US.
Analysts predict the market for RSV vaccines for adults could be worth more than $10 billion over the next decade. According to GSK, nearly 20,000 hospital deaths in Europe each year are among patients over 60 years old infected with RSV.
Source



![[UPDATE] April 30th parade rehearsal on Le Duan street in front of Independence Palace](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/18/8f2604c6bc5648d4b918bd6867d08396)
![[Photo] Prime Minister Pham Minh Chinh receives Mr. Jefferey Perlman, CEO of Warburg Pincus Group (USA)](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/18/c37781eeb50342f09d8fe6841db2426c)





























































































Comment (0)