SGGP
The US National Institutes of Health (NIH) announced the start of phase 1 clinical trials for a vaccine that prevents many new strains of influenza.
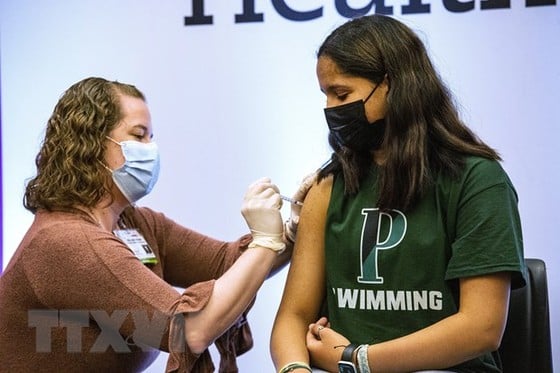 |
| Covid-19 vaccination for people in Hartford, Connecticut, USA. Photo: AFP/TTXVN |
The trial, funded by the National Institute of Allergy and Infectious Diseases (NIAID), will evaluate the investigational vaccine, FluMos-v2, for safety and ability to elicit an immune response. The NIAID-developed vaccine aims to generate antibodies against multiple strains of influenza by displaying a portion of the influenza virus’s hemagglutinin protein in repeating patterns on self-assembling nano-patterns. Exposure to these harmless pieces of viral protein helps the immune system recognize and fight the actual virus.
Animal testing results showed that the experimental vaccine generated a strong antibody response.
The new clinical trial is expected to recruit 24 healthy volunteers aged 18-50. Volunteers will receive two intramuscular injections of the FluMos-v2 vaccine, with an interval of 16 weeks between the two injections.
Most seasonal flu vaccines are designed to train the immune system against three or four different strains of the common flu, according to the NIH. Scientists hope that a universal flu vaccine could provide protection against many more strains.
Source




![[Photo] Moment of love: Myanmar people are moved to thank Vietnamese soldiers](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/3/9b2e07196eb14aa5aacb1bc9e067ae6f)
![[Photo] Special relics at the Vietnam Military History Museum associated with the heroic April 30th](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/3/a49d65b17b804e398de42bc2caba8368)

![[Photo] Comrade Khamtay Siphandone - a leader who contributed to fostering Vietnam-Laos relations](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/3/3d83ed2d26e2426fabd41862661dfff2)




















































































Comment (0)