The Drug Administration of Vietnam, Ministry of Health has just issued a decision to suspend circulation and recall nationwide a batch of Trapha deodorant powder produced by Thai Nguyen Pharmaceutical and Medical Supplies Joint Stock Company.
The recalled product batch has number 2823; manufactured on September 18, 2023; expiry date September 18, 2026; announcement number: 01/19/CBMP-TNg. Traphaco Joint Stock Company, address 75 Yen Ninh, Ba Dinh District, Hanoi is the unit responsible for bringing the product to market.
The reason for the recall was given because the test sample did not meet the quality standards for heavy metal limits.
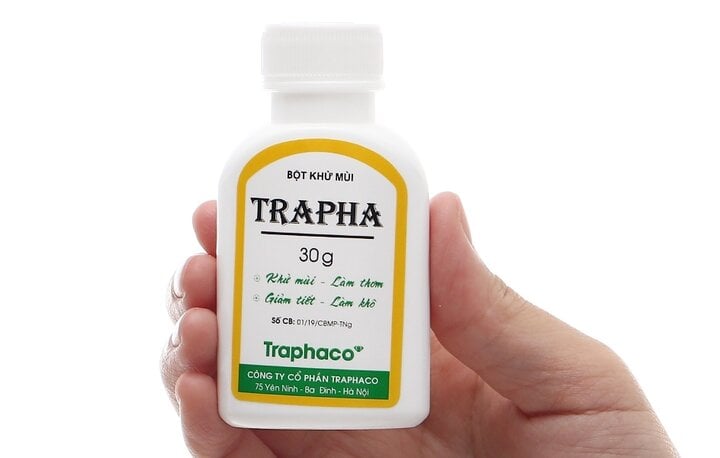
The recalled product batch has announcement number: 01/19/CBMP-TNg.
The Drug Administration of Vietnam requests the local Department of Health and the two above companies to notify businesses and cosmetic users in the area to immediately stop selling and using the above batch of Trapha Deodorant Powder - Box of 1 bottle 30g and return the product to the supplier.
The local Department of Health and the two companies shall recall and destroy the violating batch of products, inspect and supervise the units implementing this notice, and handle the violating units according to current regulations.
The Hanoi Department of Health and the Thai Nguyen Department of Health were assigned to inspect Traphaco Joint Stock Company and Thai Nguyen Pharmaceutical and Medical Supplies Joint Stock Company in their compliance with legal regulations on cosmetics management in cosmetics production and trading activities.
Previously, the Drug Administration also requested to suspend circulation and recall nationwide a newly produced batch of E-Cosmetic facial cleanser gel and a batch of Peeling acne serum.
E-Cosmetic Face wash gel product batch number: 001; production date: November 9, 2023; announcement number: 000826/23/CBMP-HCM by Long Phung Group Company Limited responsible for bringing the product to the market; branch of LAP Vietnam Pharmaceutical Company Limited manufactured. These two companies are both located in Ho Chi Minh City.
Product batch Peeling acne serum, batch number: 010623; manufacturing date: June 1, 2023; expiry date: June 1, 2026; announcement number: 2192/22/CBMP-HCM by Branch 2 of Bitechpharm Biotechnology Joint Stock Company located in Ho Chi Minh City, responsible for bringing the product to market and manufacturing.
The reason for suspending circulation and recalling the two above product batches is that the formula is not consistent with the cosmetic product declaration file.
In particular, the product E-Cosmetic Face wash gel batch 001 above contains Butylene glycol which is not included in the cosmetic product declaration file. With the product batch Peeling acne serum, in the production batch file using MC-Salicare raw materials, there are ingredients Dextrin and Nicacinamide which are not included in the cosmetic product declaration file.
The Drug Administration of Vietnam requested the health departments of 63 provinces and cities to notify businesses and cosmetic users in the area to immediately stop selling and using the above batch of E-Cosmetic Face wash gel and Peeling acne serum products and return them to the supplier. The units also recalled and destroyed the batch of violating products.
Source







































Comment (0)