The world has two approved vaccines, with efficacy of only 60 and 73%, while antiviral drugs are being urgently researched as new cases continue to rise.
Dengue fever is on the rise worldwide. The European Centre for Disease Prevention and Control counted more than 4.2 million cases as of October 2. Cases have spread to many countries, including southern Europe.
At the annual meeting of the American Society of Tropical Medicine and Hygiene, held in Chicago, Illinois, researchers shared the latest results on dengue vaccines and antiviral drugs. The perfect vaccine needs to be 90% effective in preventing the four strains of the virus, and have the same level of effectiveness in both infected and uninfected groups. No other vaccine in the world meets this standard.
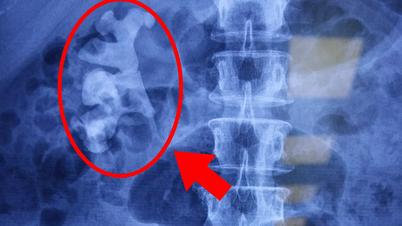
The Sanofi-made Dengvaxia vaccine has been approved in the US and has an overall efficacy rate of 60%. However, the shot is recommended for people who have had the disease. In people who have not had dengue, vaccination may increase the risk of severe disease due to antibody-dependent enhancement.
QDenga, manufactured by Takeda Corporation in Japan, has been proven safe for everyone. The overall efficacy rate is 73%. There are 4 types of viruses (also called serotypes) that cause dengue fever, including DENV-1, DENV-2, DENV-3, DENV-4; the vaccine is less effective against the DENV-3 strain and there is no convincing evidence to prevent DENV-4.
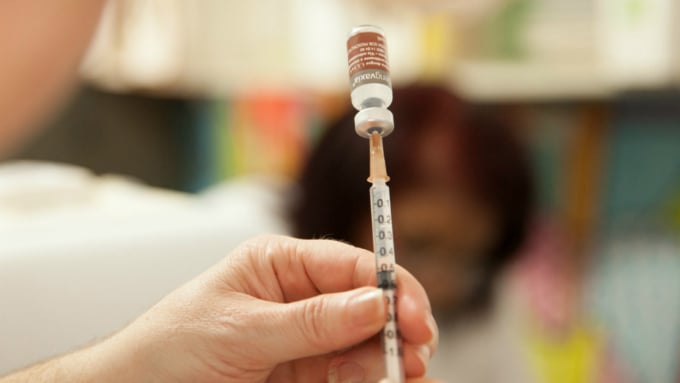
Dengvaxia vaccine prevents dengue fever. Photo: Sanofi
TV003, developed by the US National Institute of Allergy and Infectious Diseases, is being tested in Brazil on more than 16,000 people. Tracking data show the vaccine is 80% effective overall. However, scientists lack data on its effectiveness against certain serotypes, as DENV-3 and DENV-4 are not widely circulating.
Several companies are also working on antiviral drugs. Janssen Pharmaceuticals of Beerse, Belgium, has shared data on its dengue-preventing drug JNJ-1802, which is taken as a pill. Six of 10 participants who took a high dose of the experimental drug had no detectable virus in their blood, while those who took a placebo had detectable virus in their blood after five days. The virus appeared later in the group that took a low or medium dose.
Despite promising results, experts say it may be impractical and too costly to provide daily doses of the drug to all residents in an epidemic area. In addition, many people will use the drug to prevent disease when it is not necessary.
Chile (According to Nature )
Source link



![[Photo] Prime Minister Pham Minh Chinh starts construction of vital highway through Thai Binh and Nam Dinh](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/12/52d98584ccea4c8dbf7c7f7484433af5)
![[Photo] Prime Minister Pham Minh Chinh receives Swedish Minister of International Development Cooperation and Foreign Trade](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/12/ae50d0bb57584fd1bbe1cd77d9ad6d97)

![[Photo] Prime Minister Pham Minh Chinh works with the Standing Committee of Thai Binh Provincial Party Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/12/f514ab990c544e05a446f77bba59c7d1)




















































































Comment (0)