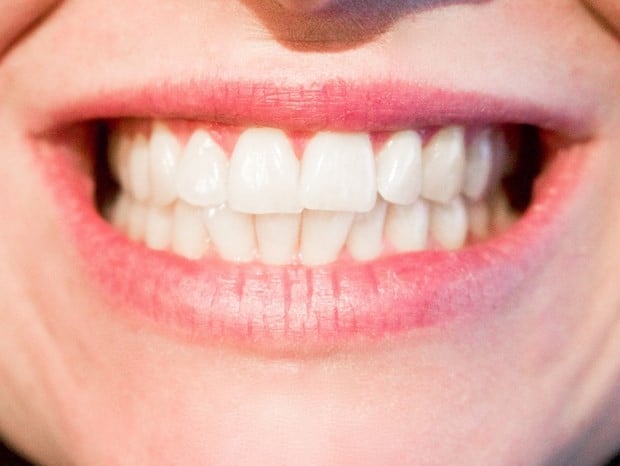
 |
| Illustration photo. (Source: Westermeier Martin Dental Care) |
If successful, the drug could hit the market around 2030 and would be the first of its kind in the world .
Clinical trials on healthy adults are expected to begin around July 2024 to confirm the drug's safety, according to a team of scientists from the startup Toregem Biopharma Co. founded by Kyoto University.
Previously, in 2018, the team had succeeded in the testing phase on mice and ferrets. The results showed that this drug helped stimulate new tooth growth in the two rodents mentioned above. Ferrets are animals that have both baby teeth and permanent teeth similar to humans.
In addition to baby and permanent teeth, most people have “tooth buds” that can develop into new teeth. However, many tooth buds fail to develop and then disappear. So the team developed an antibody drug that inhibits the protein that prevents tooth growth. In addition, the drug helps stimulate the growth of tooth buds.
In addition, from 2025, the research team plans to conduct clinical trials of this drug on children aged 2-6 years old with congenital anodontosis due to genetics or other factors. It is known that children with congenital anodontosis will grow up without some or all of their natural teeth. Such cases will be injected with the drug during the above-mentioned trial phase to stimulate tooth growth.
In addition, scientists hope to be able to use this drug in the future for adults who have lost teeth due to tooth decay.
Dr. Katsu Takahashi, co-founder of Toregem Biopharma, believes that tooth loss in children can affect the development of the jawbone. Mr. Katsu hopes that the drug will be the key to solving such problems.
Mr. Takahashi also expressed hope that in the future this product will be used as a third treatment method in medical and dental examination and treatment, besides dentures and implants.
The new drug is expected to enter clinical trials in July next year and is expected to be on the market before 2030.
Source


![[Photo] Party and State leaders attend the special art program "You are Ho Chi Minh"](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/18/6895913f94fd4c51aa4564ab14c3f250)


![[Photo] Ready for the top competitions of Vietnamese table tennis](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/18/9c547c497c5a4ade8f98c8e7d44f5a41)
![[Photo] Many young people patiently lined up under the hot sun to receive a special supplement from Nhan Dan Newspaper.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/18/6f19d322f9364f0ebb6fbfe9377842d3)




























































































Comment (0)