A team of researchers from China and New Zealand has developed a membrane that can operate for more than 5,000 hours to efficiently convert CO2 into formic acid, a useful liquid.
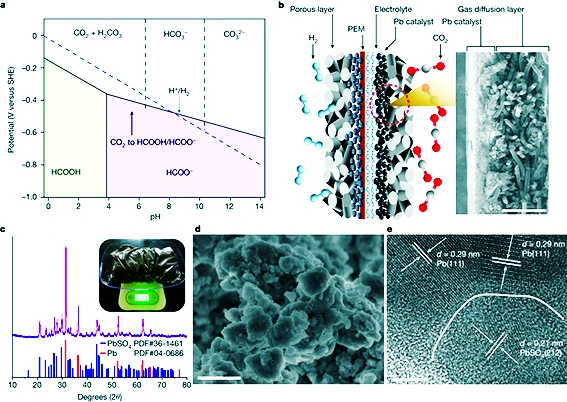
This research was published in the journal Nature. Researchers from Huazhong University of Science and Technology, China University of Science and Technology, and the University of Auckland designed a proton exchange membrane system. This sustainable CO2 conversion process is achieved using a catalyst derived from decommissioned lead-acid batteries. The electrolysis process converts CO2 into useful chemicals that could contribute to a more sustainable and carbon-neutral future.
Researcher Wen Shengfang of Huazhong University said: “This system is compatible with start/stop processes, achieving a one-time CO2 conversion efficiency of nearly 91% at a current density of 600mA cm-2 and a mobile voltage of 2.2V, and has been shown to operate continuously for over 5,200 hours. We expect this outstanding performance, achieved through the use of a robust and efficient catalyst, a stable three-phase surface, and a durable film, will help promote the development of carbon neutralization technologies.”
LAM DIEN
Source











![[Photo Gallery] Group Performance to Welcome Spring 2026](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2026/02/09/1770618453808_kkk_20260209123031.jpeg)








































































































Comment (0)