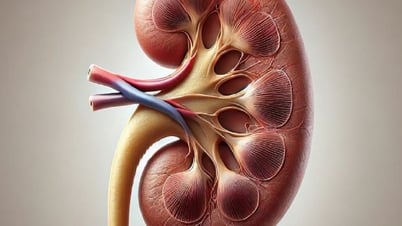
On March 25, the Food Safety Department ( Ministry of Health ) issued a warning about Kobayashi Pharmaceutical Company (Japan) recalling products that pose a risk of kidney damage.
Currently, the unit has not yet issued a Certificate of Product Declaration Registration/Certificate of Declaration of Compliance with Food Safety Regulations for the health protection food products: "Beni-koji choleste-help"; "Naishi-help plus cholesterol"; "Natto-kinase sarasara-tsubu gold" and Kobayashi Naishi Help 30 of Kobayashi Pharmaceutical Company, Japan.
For the health safety of users, the Food Safety Department warns and recommends that consumers do not buy or use products with the above information. In case these products are found circulating on the market, please notify the authorities for handling according to the provisions of law.

Recalled products.
Earlier, several media outlets reported that on March 22, Kobayashi Pharmaceutical Company reported kidney disorders and other health damage in people using their line of dietary supplements containing “beni-koji” or red fermented rice.
The Osaka-based pharmaceutical company is voluntarily recalling products containing the ingredient beni-koji and urging consumers to stop using these supplements.
The products are advertised as effective in reducing bad cholesterol thanks to their beni-koji ingredient.
So far, 13 people between the ages of 40 and 70 have been reported to have suffered health complications, of which six have been hospitalized, including two who temporarily required dialysis. Five of the patients have been discharged.
In fact, among these 3 functional foods, there is also a product sold on the Vietnamese market called Kobayashi Naishi Help 30. This product is sold at prices ranging from over 600,000 VND to over 1 million VND/box.
In Vietnam, this product is introduced with the function of converting energy during daily activities to help burn fat more easily, reducing visceral fat in people with high BMI.
Source



![[Photo] General Secretary To Lam attends the conference to review 10 years of implementing Directive No. 05 of the Politburo and evaluate the results of implementing Regulation No. 09 of the Central Public Security Party Committee.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/2f44458c655a4403acd7929dbbfa5039)
![[Photo] Close-up of Tang Long Bridge, Thu Duc City after repairing rutting](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/086736d9d11f43198f5bd8d78df9bd41)
![[Photo] President Luong Cuong presents the 40-year Party membership badge to Chief of the Office of the President Le Khanh Hai](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/a22bc55dd7bf4a2ab7e3958d32282c15)
![[Photo] Panorama of the Opening Ceremony of the 43rd Nhan Dan Newspaper National Table Tennis Championship](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/5e22950340b941309280448198bcf1d9)
























![[Photo] Prime Minister Pham Minh Chinh inspects the progress of the National Exhibition and Fair Center project](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/35189ac8807140d897ad2b7d2583fbae)



























































![[VIDEO] - Enhancing the value of Quang Nam OCOP products through trade connections](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/17/5be5b5fff1f14914986fad159097a677)





Comment (0)