AstraZeneca has proposed terminating contracts or ending approval for the use of the Covid-19 vaccine for urgent needs in countries, instead of recalling the vaccine globally.
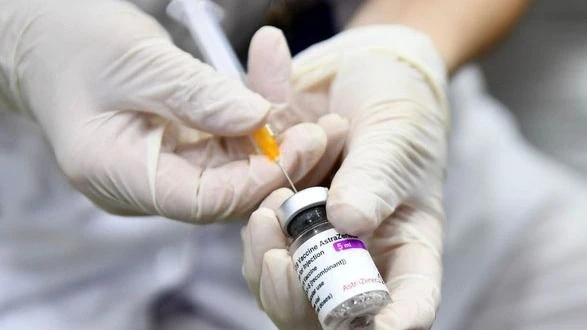
On May 9, a representative of the Drug Administration of Vietnam, Ministry of Health, said that the unit had received a notice from AstraZeneca Pharmaceutical Company (UK) requesting to terminate the approval of the use of AstraZeneca vaccine to prevent Covid-19 for urgent needs in Vietnam because the epidemic is no longer in the urgent phase.
"The process of licensing this vaccine is the same as the process of requesting to terminate approval for use. We also have to submit it to the council for review and comments, after which the management agency will make a decision on terminating use," the representative of the Drug Administration of Vietnam stated. At the same time, he added that AstraZeneca proposed to terminate the contract or terminate approval for the use of the Covid-19 vaccine for urgent needs in countries, not to recall this vaccine globally.
For Vietnam, since July 2023, there has been no more AstraZeneca Covid-19 vaccine. Because the number of AstraZeneca Covid-19 vaccines previously licensed for circulation has expired. After that, AstraZeneca Covid-19 vaccines will no longer be imported into Vietnam.
Therefore, after the above announcement from AstraZeneca, in the future, if this vaccine is used again, it will be necessary to redo the import procedures, allowing the vaccine to be used in accordance with regulations.

Previously, the Ministry of Health licensed AstraZeneca's Covid-19 vaccine to be circulated in Vietnam and at the time of licensing, this vaccine had been licensed for emergency use in 181 countries and territories. At the same time, this is also the vaccine that the World Health Organization has put on the list of emergency use.
In Vietnam, during the Covid-19 pandemic, in addition to the 30 million doses of Covid-19 vaccine ordered from AstraZeneca, Vietnam also received AstraZeneca's Covid-19 vaccine sporadically through sponsorship programs.
Most recently, in February 2023, the National Institute of Hygiene and Epidemiology allocated more than 800,000 doses of AstraZeneca Covid-19 vaccine with an expiry date of July 2023 to localities to deploy booster vaccinations for people aged 18 and over.
In response to the news that the AstraZeneca Covid-19 vaccine may cause blood clotting side effects, the Ministry of Health advised people not to panic or worry, because side effects of the AstraZeneca Covid-19 vaccine are very rare and only occur within 28 days of use. Meanwhile, most people have been vaccinated past this period.
NATIONAL ESTABLISHMENT
Source: https://www.sggp.org.vn/astrazeneca-de-nghi-viet-nam-cham-dut-phe-duyet-su-dung-vaccine-ngua-covid-19-post739151.html


![[Photo] Vietnam shines at Paris International Fair 2025 with cultural and culinary colors](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/5/4/74b16c2a197a42eb97597414009d4eb8)
![[Photo] Bus station begins to get crowded welcoming people returning to the capital after 5 days of holiday](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/5/4/c3b37b336a0a450a983a0b09188c2fe6)


![[Photo] General Secretary To Lam receives Sri Lankan President Anura Kumara Dissanayaka](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/5/4/75feee4ea0c14825819a8b7ad25518d8)


















































































![[Video]. Building OCOP products based on local strengths](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/5/3/61677e8b3a364110b271e7b15ed91b3f)



Comment (0)