In a trial on brain tumor patients, the SurVaxM vaccine was shown to nearly double survival time and can eliminate and prevent tumor recurrence.
John Wishman (61 years old, New York, USA) was diagnosed with glioblastoma in the fall of 2020, the most dangerous form of brain cancer, with an average survival time of only 12-18 months. However, after two and a half years, he is still traveling and enjoying life.
Wishman said the reason was because he had used an experimental vaccine that was shown to slow tumor growth. The vaccine, called SurVaxM, targets the survivin protein found in tumors, which helps cancer cells survive. The manufacturer argues that removing survivin could cause cancer cells to die. Wishman received the vaccine through an expanded program that allows seriously ill people to get access to experimental drugs.
Tracey Kassman, 65, also enrolled in the trial in April 2022, three months after she was diagnosed with glioblastoma. She received her first shot that month, and now gets it every two months. But because the trial is randomized, Kassman and her followers don’t know whether they’re getting the vaccine or a placebo.
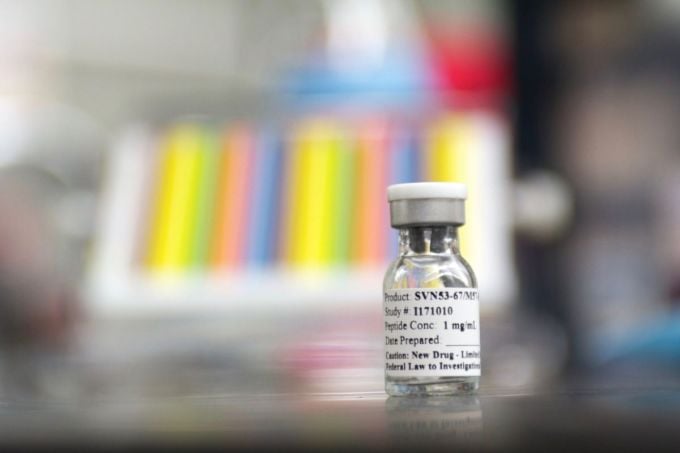
Vial of experimental SurVaxM vaccine. Photo: Roswell Park
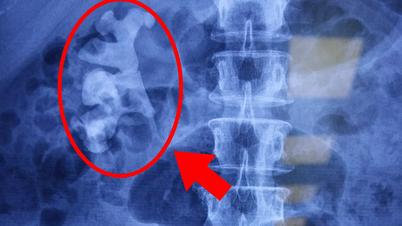
Glioblastoma grows rapidly and has a tendency to invade other parts of the brain and spinal cord when it is discovered. The disease is likened to an octopus's tentacles reaching into different parts of the brain, and it is impossible to surgically remove the entire tumor. Treatments include surgery, chemotherapy, and radiation, but the tumor often recurs.
More than 14,000 people in the United States were diagnosed with the disease last year, accounting for nearly half of all malignant brain tumors, according to Tom Halkin, a spokesman for the National Brain Tumor Association. The disease has a five-year survival rate of just 6.8 percent.
In the first clinical trial, SurVaxM extended the median survival of 63 brain cancer patients by 26 months. The vaccine maker is now recruiting a larger number of patients, up to 270, to confirm the results. The trial, which is scheduled to take place at more than 10 sites in the US and China, will compare the vaccine with patients receiving usual care.
Michael Ciesielski, CEO of MimiVax, the vaccine's maker, said SurVaxM works by training the immune system to attack cancer cells, so when a tumor recurs, the body can eliminate it and prevent new tumors from growing.
Trial participants will undergo surgery to remove as much of the tumor as possible, followed by radiation and chemotherapy using the drug temozolomide, said Dr. Robert Fenstermaker, chief of neurosurgery at Roswell Park Comprehensive Cancer Center and principal investigator of SurVaxM.
“About a month after radiation, while the radiation is still working, we want to start vaccinating because that's when the immune system has rejuvenated,” says Dr. Fenstermaker.
The vaccine is given in the arm, similar to a flu or Covid-19 vaccine, in four doses, spread over two months, followed by a booster every two months. Trial participants will be given either the actual vaccine or a placebo, and then have their brains checked every two months to monitor for signs of progress.
This isn’t the first time scientists have tried to find a way to delay the recurrence of glioblastoma. Other cancer vaccines have targeted survivin, but none have made it past mid- to late-stage clinical trials, according to Ciesielski.
Dr. Alyx Porter, a neuro-oncologist at the Mayo Clinic in Phoenix, said the approach is different from previous trials. Targeted therapies such as checkpoint inhibitors, for example, have been used for years and have improved survival in patients with a variety of cancers, including breast and lung cancer. But these drugs are less effective against brain tumors because they cannot cross the barrier that keeps foreign substances from entering the brain. The new vaccine would create antibodies that could reach the brain. However, the evidence needs to be solidified.
According to Ciesielski, the results of the phase 2b trial are not expected until mid-2024, and the trial may not be completed for another 18 to 24 months. If successful, the company will move on to a phase 3 clinical trial.
Fenstermaker said the drug appears to be safe so far. Side effects of the vaccine include fever, itching, rash, and muscle pain. Ciesielski said the company is also looking to use the vaccine for other types of cancer, including multiple myeloma and neuroendocrine tumors, a rare form of cancer that can develop anywhere there are neuroendocrine cells, such as the lungs and pancreas.
Chile (According to NBC News )
Source link


![[Photo] President Luong Cuong presents the decision to appoint Deputy Head of the Office of the President](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/8/501f8ee192f3476ab9f7579c57b423ad)
![[Photo] Prime Minister Pham Minh Chinh meets with the Policy Advisory Council on Private Economic Development](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/8/387da60b85cc489ab2aed8442fc3b14a)


![[Photo] General Secretary concludes visit to Azerbaijan, departs for visit to Russian Federation](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/8/7a135ad280314b66917ad278ce0e26fa)
![[Photo] National Assembly Chairman Tran Thanh Man chairs the meeting of the Subcommittee on Documents of the First National Assembly Party Congress](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/8/72b19a73d94a4affab411fd8c87f4f8d)




















































![[Photo] Prime Minister Pham Minh Chinh talks on the phone with Singaporean Prime Minister Lawrence Wong](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/8/e2eab082d9bc4fc4a360b28fa0ab94de)































Comment (0)