On July 13, the Ho Chi Minh City Department of Health announced the results of the inspection and assessment of vaccination safety at vaccination facilities of FPT Long Chau Pharmaceutical Joint Stock Company.
Accordingly, in the city, there are 21 vaccination facilities belonging to FPT Long Chau Pharmaceutical Joint Stock Company in 12 districts of Thu Duc City.
However, after review, FPT Long Chau Pharmaceutical Joint Stock Company requested to remove 1 facility at Long Chau 60 central business location (address 356 Pham Hung, Ward 5, District 8) from the list of facilities declared eligible for vaccination.
The reason is that during the process of evaluating operations, the Company researched to find other more suitable facilities. Thus, up to now, in Ho Chi Minh City, there are still 20 vaccination facilities belonging to the above Company.
In the past time, the Health Departments have coordinated with the District Health Centers, Thu Duc City to inspect and examine vaccination activities in the management area, according to the provisions of Circular No. 34/2018/TT-BYT dated November 12, 2018 of the Ministry of Health.
Through reports from the Health Department, the conditions for vaccination implementation are maintained.
However, some facilities still have shortcomings that need to be overcome, such as: facilities do not arrange facilities appropriately, ensure one-way procedures, are not equipped with backup generators, fire prevention and fighting equipment, are not equipped with a warning system with bells and lights when there is a problem with vaccine storage temperature (warnings are only sent by text messages and emails), vaccines stored in refrigerators do not ensure distance...
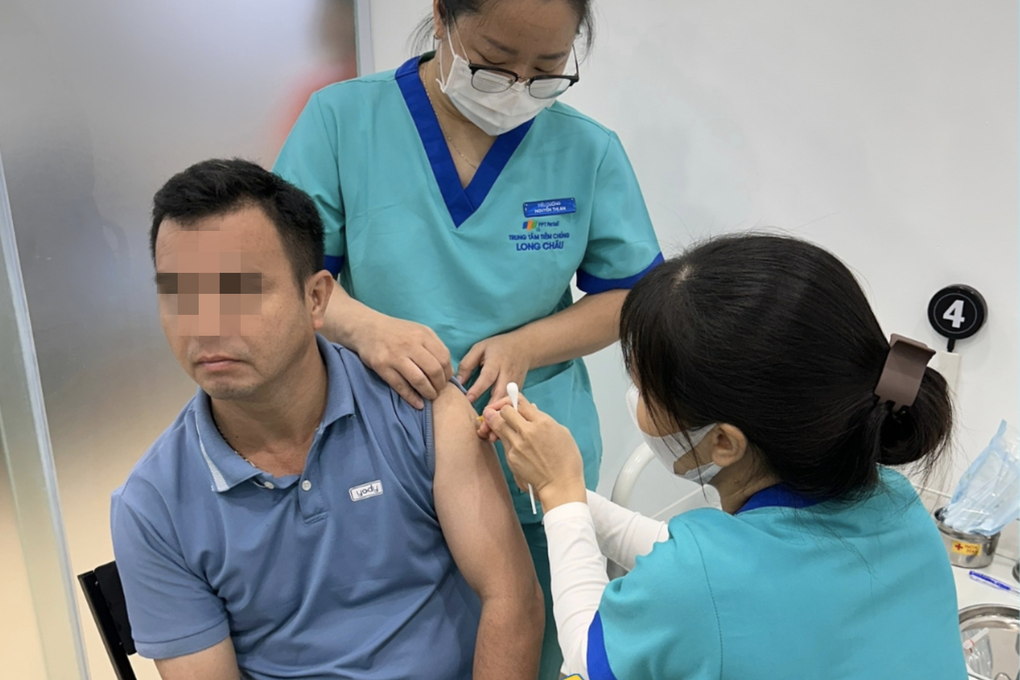
A case of vaccination at the vaccination facility of FPT Long Chau Pharmaceutical Joint Stock Company (Photo: LC).
The Health Department has made a record and the Company is committed to correcting the above content.
Regarding the two cases of anaphylaxis after vaccination at two facilities under FPT Long Chau, the Department of Health said that on July 3-4, the agency received a report of a grade 3 anaphylaxis case after vaccination with Twinrix vaccine (to prevent hepatitis AB) and Bexsero vaccine (to prevent group B meningococcal disease); a grade 2 anaphylaxis case after vaccination with Twinrix vaccine from two different vaccination facilities in the Long Chau system.
These are also the first two cases reported since the FPT Long Chau vaccination facilities began operating in Ho Chi Minh City. The anaphylactic reactions of both cases occurred within 30 minutes of post-vaccination monitoring at the facility and were treated on-site by the facility, then transferred to the nearest hospital for further monitoring and treatment.
Currently, both patients are stable and discharged from the hospital. The vaccination facility has reported the case of serious adverse events after vaccination to the Department of Health according to regulations.
Source: https://dantri.com.vn/suc-khoe/tphcm-cong-bo-ket-qua-kiem-tra-cac-co-so-tiem-chung-cua-fpt-long-chau-20240713132515141.htm




![[Photo] Prime Minister Pham Minh Chinh chairs a meeting of the Steering Committee for key projects in the transport sector.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/10/0f4a774f29ce4699b015316413a1d09e)
![[Photo] General Secretary To Lam holds a brief meeting with Russian President Vladimir Putin](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/10/bfaa3ffbc920467893367c80b68984c6)






















































































Comment (0)