The US Food and Drug Administration (FDA) approved a revolutionary gene-editing therapy to treat sickle cell disease.
The FDA decision was made on December 9. The two approved therapies are called Casgevy, which is based on the Nobel Prize-winning CRISPR gene-editing technology, and Lyfgenia, which uses a harmless virus to edit genes.
Casgevy is produced by two companies, Vertex Pharmaceuticals (USA) and CRISPR Therapeutics (Switzerland). The drug has the effect of repairing defective genes in stem cells of patients aged 12 and older with sickle cell disease and thalassemia.
Both diseases are caused by defects in the genes that carry hemoglobin. Gene therapy treatments typically cost millions of dollars, out of reach for many patients. The manufacturer, Vertex Pharmaceuticals, is working to keep the price as low as possible.
The FDA approval comes after the UK's Medicines and Healthcare products Regulatory Agency (MHRA) approved the therapy in November.
Lyfgenia, meanwhile, uses gene transfer to edit genes. It alters a patient’s blood stem cells to produce hemoglobin derived from the gene. When added to red blood cells, the hemoglobin reduces the risk of sickle cell disease, according to the FDA.
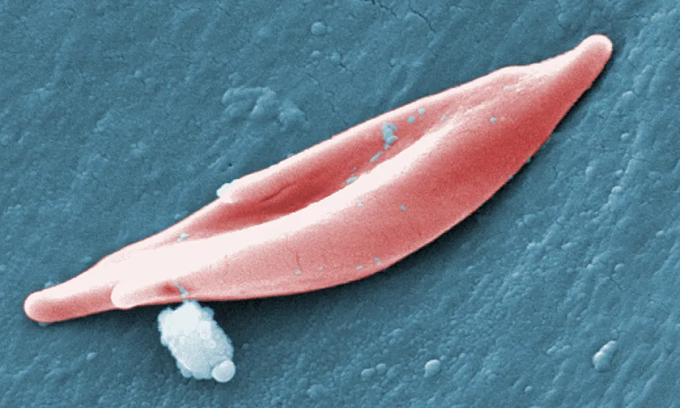
A sickle-shaped red blood cell. Photo: CDC
"These treatments represent major advances in the field of gene therapy for patients with sickle cell disease. Their potential to change lives is enormous," said Peter Marks, director of the FDA's Center for Biologics Evaluation and Research.
Gene therapy makes treatment more targeted and effective, especially for people with rare diseases who have limited treatment options, according to FDA's Dr. Nicole Verdun.
The FDA is “excited to advance this field,” she added, because they help individuals whose lives are severely disrupted by the disease.
The agency’s approval means about 16,000 people with sickle cell disease will have a potential treatment option. The disease affects about 100,000 Americans and more than 7.7 million people worldwide, mostly of African and Caribbean descent. It is a rare, debilitating and life-threatening blood disorder, and many have unmet treatment needs.
Sickle cell patients have elongated, sickle-shaped blood cells instead of the typical concave-shaped round ones. The misshapen cells get stuck in blood vessels, blocking oxygen supply, causing patients to suffer severe pain, possibly stroke, and organ damage.
Thuc Linh (According to AFP, AP )
Source link






![[Photo] Bustling construction at key national traffic construction sites](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/5/2/a99d56a8d6774aeab19bfccd372dc3e9)






















![[Photo] "Lovely" moments on the 30/4 holiday](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/5/1/26d5d698f36b498287397db9e2f9d16c)

![[Photo] Binh Thuan organizes many special festivals on the occasion of April 30 and May 1](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/5/1/5180af1d979642468ef6a3a9755d8d51)































































Comment (0)