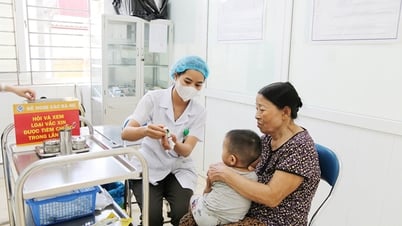
The dengue fever vaccine produced by Takeda (Qdenga vaccine) has just been licensed by the Ministry of Health for circulation in Vietnam and is indicated for people aged 4 years and older without the need for testing before vaccination.
On May 17, a representative of the Ho Chi Minh City Department of Health said that the dengue fever vaccine that has just been approved by the Ministry of Health is expected to be available at some vaccination centers in the country from September 2024. Thus, from now on, to prevent dengue fever, in addition to preventive measures by controlling disease vectors such as killing mosquitoes, larvae, and pupae, Vietnam has a new "weapon", the Qdenga vaccine.

It is expected that the dengue fever vaccine will be deployed at nearly 200 VNVC Centers.
However, to reduce the morbidity and mortality rate due to dengue fever, it is necessary to continue to maintain and promote the measures being implemented such as improving the capacity for diagnosis, treatment and care at all health levels, and controlling disease vectors such as eliminating mosquitoes, larvae and pupae.
Doctor Bach Thi Chinh, Medical Director of VNVC Vaccination System, said that VNVC Vaccination System is a comprehensive strategic partner of Takeda, and the unit has also negotiated to make efforts to soon bring this important vaccine to serve people in the system of nearly 200 VNVC Centers nationwide.
According to Associate Professor, Dr. Pham Quang Thai, Deputy Head of the Department of Infectious Disease Control, National Institute of Hygiene and Epidemiology, dengue fever is very different from other infectious diseases, because this is a disease that has the potential to spread into an epidemic in a country, regardless of age or past infection status of the people.
Treatment costs also affect household income, while the disease also leaves long-term psychological effects on individuals and impacts on social security. Along with climate change, the increasing number of dengue fever patients has put pressure and overloaded medical facilities in the country.
“In addition to vector control, early detection of outbreaks, appropriate treatment organization and strengthening facilities and equipment to manage dengue fever and reduce mortality rates, the Ministry of Health's approval of the dengue vaccine is a specific preventive measure, helping to protect Vietnamese people from dengue fever,” Associate Professor, Dr. Pham Quang Thai emphasized.
According to the manufacturer, the Qdenga vaccine can protect against all four serotypes of the dengue virus (DEN-1, DEN-2, DEN-3 and DEN-4), indicated for the prevention of dengue fever in people 4 years of age and older. Especially in areas where dengue is endemic without the need for testing to determine previous infection with the dengue virus. The vaccination schedule consists of 2 doses, 3 months apart.
The clinical efficacy of Qdenga has been evaluated in phase 3, double-blind, randomized, placebo-controlled clinical trials conducted in many countries with a duration of protection of up to 4.5 years after the second dose. However, a booster dose to boost immunity is not recommended. After vaccination, some people may experience mild side effects such as pain, swelling at the injection site and mild fever.
The development of the Qdenga vaccine began in 2010 and went through several phases of clinical trials before being approved. The Qdenga vaccine has received priority review from the US Food and Drug Administration (FDA) and has been evaluated by the European Medicines Agency (EMA).
The dengue vaccine has also been approved in more than 30 countries, including the European Union, the United Kingdom, Brazil, Argentina, Indonesia, Thailand and Malaysia. It has also been approved and used in national immunization programs in Brazil and Argentina.
Source


![[Photo] Prime Minister Pham Minh Chinh starts construction of vital highway through Thai Binh and Nam Dinh](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/12/52d98584ccea4c8dbf7c7f7484433af5)

![[Photo] Buddha's Birthday 2025: Honoring the message of love, wisdom, and tolerance](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/12/8cd2a70beb264374b41fc5d36add6c3d)























![[Photo] General Secretary To Lam meets and expresses gratitude to Vietnam's Belarusian friends](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/11/c515ee2054c54a87aa8a7cb520f2fa6e)






























































Comment (0)