In the context of Vietnam's economy increasingly integrating deeply into the global value chain and the emergence of situations causing supply chain disruptions (pandemics, wars, trade protectionism, etc.), it shows the importance of being self-sufficient in a part of essential raw materials used to produce drugs to ensure social security - health care for the people. Developing the pharmaceutical industry - one of the three key sub-sectors of pharmaceutical production - is considered an important driving force in the modernization and enhancement of national competitiveness. The Pharmaceutical Industry Development Program to 2030, with a vision to 2045, has been approved by the Prime Minister, not only demonstrating the State's strategic orientation in improving the quality of pharmaceutical products and self-sufficiency in raw material supply, but also a driving force for Vietnam to become an ASEAN pharmaceutical center.
Targets towards 2030 and 2045
The program is built with a long-term vision, aiming to transform the Vietnamese pharmaceutical industry from a state of dependence on imported raw materials to a modern production ecosystem, meeting domestic demand and participating deeply in the global value chain. Specifically, the strategic goals of the program include:
- Increasing domestic raw material production: The goal by 2030 is to strive to meet 20% of raw material demand for pharmaceutical production, while ensuring 50% of raw material demand for functional food and pharmaceutical cosmetics production. This is an essential step to reduce dependence on imported supplies and ensure supply security in the context of global economic fluctuations.
- Promoting exports and increasing added value: The program aims to accelerate the export growth of natural pharmaceutical products such as pharmaceutical substances, quantitative extracts and essential oils rich in active ingredients with an average growth rate of 10% per year. At the same time, focusing on research, technology transfer and application of advanced technologies will help improve product quality, creating higher economic value for the industry.
- Building a modern pharmaceutical industry: The vision for 2045 is to turn Vietnam's pharmaceutical industry into a modern, high-tech industry with strong competitiveness in the international market. The growth rate of industrial production in the pharmaceutical industry is 8-11%/year. This not only contributes to ensuring the supply of safe, quality pharmaceuticals but also acts as a driving force for the country's sustainable economic growth.
These goals demonstrate the State's commitment to modernizing production, transforming technology and building a strong domestic pharmaceutical industry, contributing to ensuring public health and developing the national economy.
Role of the Ministry of Industry and Trade
Within the framework of the Program implementation, the Ministry of Industry and Trade plays a key role, not only as a policy maker but also as a bridge between the State and enterprises, between domestic resources and international partners. The role of the Ministry of Industry and Trade is demonstrated through the following key tasks:
1. Taking the lead in developing and implementing the Program: The Ministry of Industry and Trade is responsible for concentrating resources and closely coordinating with the Ministry of Health, the Ministry of Science and Technology, the Ministry of Agriculture and Environment , and the Ministry of Finance to develop a legal framework and mechanism for implementing the program. This coordination helps ensure that all regulations, technical standards, and incentive policies are issued synchronously, creating a favorable environment for domestic enterprises and multinational pharmaceutical corporations to invest and do business in Vietnam.
2. Administrative reform and investment facilitation: The Ministry has and will closely coordinate with relevant ministries and branches to review, amend and supplement legal regulations on investment, tax and credit to create specific mechanisms for pharmaceutical production projects. This is to attract investment in special fields that require large capital and high technology such as the pharmaceutical industry.
3. Promoting international cooperation and technology transfer: Taking advantage of the free trade agreements that Vietnam has signed, the Ministry of Industry and Trade will promote the expansion of cooperation with multinational corporations and international partners. Thereby, creating conditions for domestic enterprises to have the opportunity to learn from experience, access modern technology and expand export markets, contributing to shaping the position of the pharmaceutical industry in the international arena.
4. Ensuring safe supply and developing value chains: The Ministry of Industry and Trade coordinates with the Ministry of Health and localities to build a system for producing pharmaceutical raw materials that meet standards, control quality and ensure stable supply. Developing a value chain from medicinal material exploitation, pharmaceutical chemical production to product distribution will help reduce dependence on imported supplies, while enhancing the self-sufficiency of the Vietnamese pharmaceutical industry. In fact, from domestic medicinal plant sources, many important active ingredients can be extracted and refined to serve the production of pharmaceutical raw materials. For example, Rutin in Sophora japonica flowers helps produce drugs that strengthen blood vessels, prevent and treat bleeding diseases. Curcumin from turmeric has the effect of preventing tumors, supporting the treatment of cancer and duodenal ulcers. Shikimic acid in star anise essence is the raw material for producing Oseltamivir phosphate, an important active ingredient in drugs to prevent influenza A/H5N1 and H1N1 viruses. Artemisinin from Artemisia annua serves the production of antimalarial drugs (DHA, artesunate, artemeter), not only meeting domestic demand but also having export potential. The Ministry of Industry and Trade is in charge of researching and developing a product brand program for at least 05 typical pharmaceutical products.
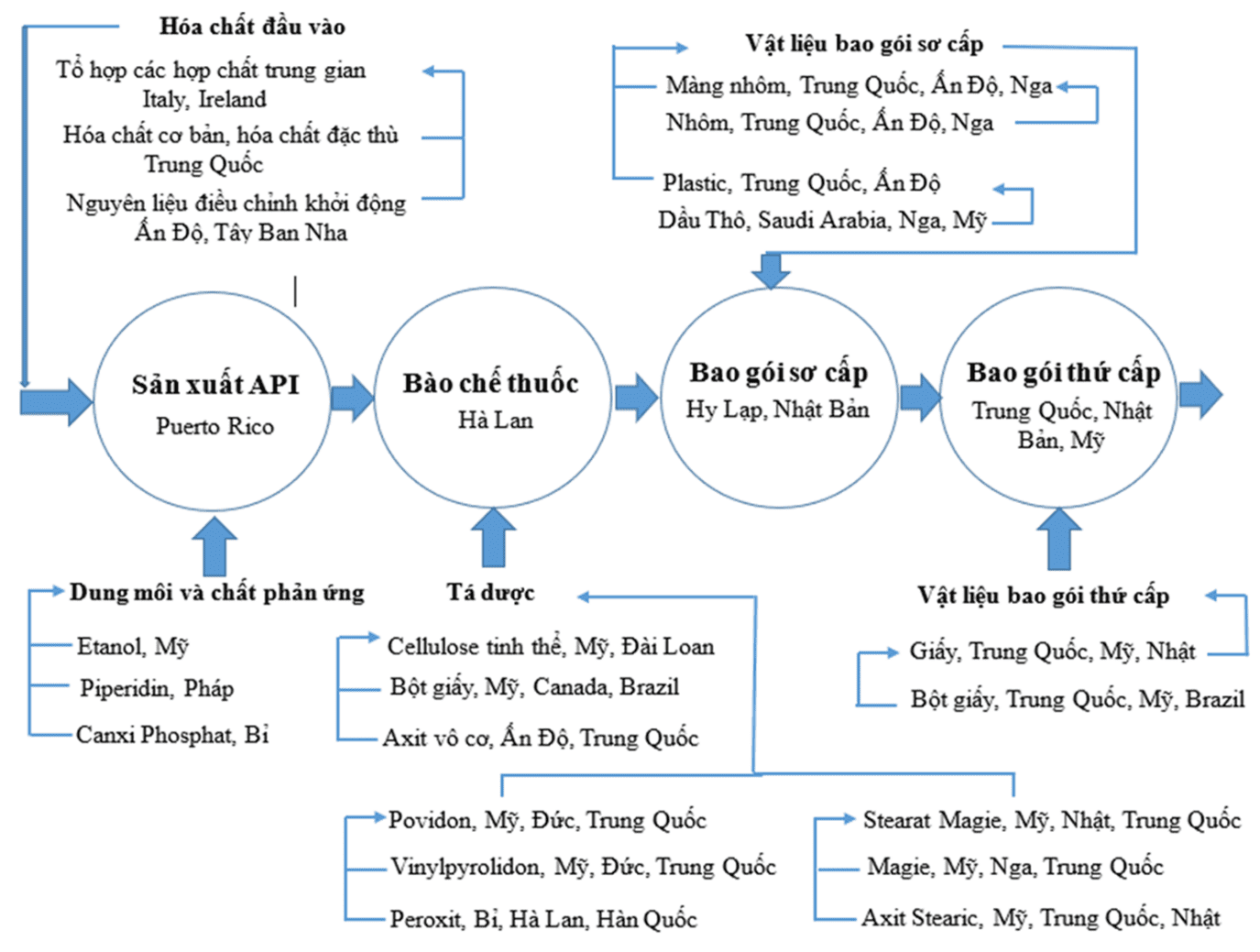
The pharmaceutical value chain includes many stages, from API production, drug formulation to packaging and distribution, with input materials coming from many countries. Developing a domestic supply chain will help reduce import dependence and improve the industry's self-sufficiency. The diagram below illustrates a typical pharmaceutical chemical production chain, demonstrating the complexity of the industry and the urgent need for localization of raw materials.
- Support for training and human resource development: Technology transfer and application of advanced technologies require a high-quality workforce. The Ministry of Industry and Trade promotes links between universities, research institutes and enterprises to train and develop experts, engineers and scientists, ensuring human resources meet modern production requirements and participate in product research and development.
These activities affirm the key role of the Ministry of Industry and Trade in orienting and promoting the development of the pharmaceutical industry, contributing to creating momentum for the comprehensive transformation of the Vietnamese economy.
Challenges to be addressed
Despite preferential policies and extensive attention from the State, the Vietnamese pharmaceutical industry is still facing many challenges that need to be overcome to achieve the set goals. These challenges include:
1. Challenges in production capacity and technology: Most pharmaceutical manufacturing enterprises are currently small in scale, with outdated equipment and production processes that do not meet international standards. This reduces competitiveness and limits the industry's development potential. Upgrading production facilities to meet GMP and international standards is still facing many difficulties due to limited investment and modern technology. Delays in the process of production modernization directly affect product quality and market expansion.
2. Challenges in human resources and training: To transform into high-tech production, the pharmaceutical industry needs a team of highly qualified experts, engineers and scientists. Currently, high-quality human resources in this field are still limited, affecting the research and application of advanced technology. The lack of synchronization between training facilities, research institutes and enterprises causes difficulties in technology transfer and human resource training according to modern production requirements.
3. Challenges in raw materials and supply chains: A large part of pharmaceutical raw materials such as active ingredients and excipients still have to be imported from countries such as China and India, increasing supply risks and affecting production stability. Developing a domestic raw material production system that meets standards is an urgent issue that needs to be addressed. The value chain system from medicinal material production, raw material processing, pharmaceutical production to product distribution and consumption still has many weaknesses. This not only reduces production efficiency but also affects the quality of the final product.
4. Challenges in investment mechanisms, policies and environment: Although the State has issued many legal documents and investment incentive policies, the implementation and coordination between agencies and ministries still have some inconsistencies. This hinders the process of attracting investment and developing domestic enterprises. Investment in the pharmaceutical sector requires large capital, especially for the construction of GMP-standard production facilities, research and technology transfer. Capital from the state budget and private investment sources need to be mobilized more effectively to meet the needs of technology transformation.
5. Challenges of international integration and global competition: Foreign enterprises with large scale and modern technology are dominating the international market. To compete, the Vietnamese pharmaceutical industry needs to have a separate development strategy, focusing on the advantages of medicinal material resources and creativity in product research and development. Although there are many opportunities to learn from countries with developed pharmaceutical industries, technology transfer and international cooperation still face legal, economic and corporate culture barriers. This requires adjustments and creativity in policies and cooperation mechanisms to maximize the advantages from outside.
Directions and Solutions
To overcome the above challenges, the Ministry of Industry and Trade, together with relevant ministries and sectors, has proposed a number of key solutions to create a breakthrough in the development of the pharmaceutical industry:
- Promoting administrative reform and preferential policies: Simplifying administrative procedures, improving the investment environment and facilitating domestic enterprises' access to capital are key factors in enhancing the industry's production capacity and competitiveness.
- Strengthening training links and technology transfer: Cooperation between universities, research institutes and enterprises needs to be strongly promoted. Attracting foreign experts and scientists with international experience will contribute to improving the quality of human resources and applying advanced technologies to production.
- Developing domestic value chains: Building a supply chain system from medicinal material production to pharmaceutical processing and production must be done synchronously, ensuring quality standards and continuity of raw material sources. This is the decisive factor for the autonomy and stability of pharmaceutical production in Vietnam.
- Promote international cooperation: Take advantage of free trade agreements and participate in international forums to help domestic enterprises expand markets, transfer technology and learn from advanced countries in the pharmaceutical chemistry field.
- Investment in infrastructure and industrial park construction: The construction of specialized pharmaceutical industrial parks and high-tech parks meeting international standards will be an important driving force in attracting investment and increasing production scale, creating momentum for the sustainable development of the industry.
The Pharmaceutical Industry Development Program to 2030, with a vision to 2045, is not only a roadmap for the transformation of the pharmaceutical industry but also a clear expression of the State's commitment to building a modern, self-reliant and competitive industry in the international arena. Under the leadership of the Ministry of Industry and Trade, with close coordination of relevant ministries and sectors, the Program has set specific strategic goals to increase domestic raw material production, transfer advanced technology and improve product quality. Thereby, Vietnam will gradually reduce its dependence on imported supplies and affirm its national brand in the global value chain.
However, to achieve these ambitious goals, the pharmaceutical industry still needs to overcome many challenges – from upgrading production capacity, improving modernization processes, training high-quality human resources to building a complete domestic supply chain and attracting essential investment capital. The coordinated efforts of the “four houses” including management agencies, businesses, researchers and farmers will be the key to opening the door to sustainable development for the industry.
In the future, when the pharmaceutical industry transforms into a high-tech industry with products that meet international standards, the development of the industry will not only create great economic value but also improve the quality of life of the people. This is the ultimate goal that the program is aiming for - a modern, self-reliant and integrated Vietnam, affirming its position on the global economic map.
Source: https://moit.gov.vn/tin-tuc/phat-trien-cong-nghiep/chuong-trinh-phat-trien-cong-nghiep-hoa-duoc-den-nam-2030-tam-nhin-den-nam-2045.html




![[Photo] Close-up of Tang Long Bridge, Thu Duc City after repairing rutting](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/086736d9d11f43198f5bd8d78df9bd41)
![[Photo] President Luong Cuong presents the 40-year Party membership badge to Chief of the Office of the President Le Khanh Hai](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/a22bc55dd7bf4a2ab7e3958d32282c15)
![[Photo] Panorama of the Opening Ceremony of the 43rd Nhan Dan Newspaper National Table Tennis Championship](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/5e22950340b941309280448198bcf1d9)
![[Photo] General Secretary To Lam attends the conference to review 10 years of implementing Directive No. 05 of the Politburo and evaluate the results of implementing Regulation No. 09 of the Central Public Security Party Committee.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/2f44458c655a4403acd7929dbbfa5039)




























![[Photo] Prime Minister Pham Minh Chinh inspects the progress of the National Exhibition and Fair Center project](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/35189ac8807140d897ad2b7d2583fbae)




















































![[VIDEO] - Enhancing the value of Quang Nam OCOP products through trade connections](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/17/5be5b5fff1f14914986fad159097a677)



Comment (0)