On September 28, the VNVC Vaccination Center System organized a strategic cooperation signing ceremony with Takeda Pharmaceuticals (Asia Pacific) Co., Ltd., a member of the Takeda Group of Japan.
The cooperation between Vietnam's leading vaccination center system and the world's leading pharmaceutical group Takeda is expected to soon bring opportunities to access and apply dengue fever prevention solutions, especially dengue fever vaccines, to the Vietnamese people.
At the signing ceremony, the two sides agreed to implement many important activities, affirming the sustainable development strategy with the goal of training, scientific research, etc. to promote awareness and improve professional knowledge for medical staff on measures to prevent dengue fever, including dengue fever vaccine.
Attending the signing ceremony, Dr. Nguyen Ngo Quang, Deputy Director of the Department of Science, Technology and Training (Ministry of Health) highly appreciated the cooperation between Takeda and VNVC - Vietnam's leading complete vaccination system. Mr. Quang expected that the cooperation between Takeda and VNVC will bring practical benefits to the people in the near future.
"We hope that Vietnamese people will have access to dengue fever vaccine as soon as possible," said Mr. Quang.
With many years of experience in the field of preventive medicine and vaccination, Associate Professor, Dr. Tran Dac Phu, former Director of the Department of Preventive Medicine, Senior Advisor of the Vietnam Center for Public Health Emergency Response, shared that he has witnessed many outbreaks and deaths caused by dengue fever and understands better than anyone the role of vaccines in preventing many infectious diseases.
He highly appreciated the superior vaccination model of VNVC and expressed his joy at the growth, reputation and quality of VNVC when there are currently more than 130 vaccination centers nationwide, helping people have easier access to new vaccines circulating in the world.
He hopes that Vietnam will soon have a dengue fever vaccine, helping to reduce the number of cases and deaths caused by the disease, contributing to sustainable control of this epidemic in Vietnam.

Representatives of Takeda Vietnam Company and representatives of VNVC Vaccination System signed a memorandum of understanding on strategic cooperation on September 28 in Ho Chi Minh City. Photo: Thanh Tung
At the signing ceremony, Mr. Ngo Chi Dung, Chairman of the Board of Directors - General Director of VNVC Vaccination Center System shared that with comprehensive capacity and experience in preservation and being a strategic partner of many major vaccine companies in the world, VNVC is always interested in finding and ready to cooperate with manufacturers to promptly bring new generation vaccines to prevent diseases such as dengue fever, herpes zoster, hand, foot and mouth disease, hepatitis B... to serve the Vietnamese people.
"VNVC has prepared the best conditions to cooperate with Takeda Group to raise awareness about dengue fever. The cooperation event opens up the prospect of applying advanced measures to prevent dengue fever, especially with dengue fever vaccine in the future in Vietnam," Mr. Dung expected.
Speaking at the signing ceremony, Ms. Katharina Geppert - Chief Representative of Takeda Vietnam shared: "We are very pleased to cooperate with VNVC to bring Takeda's high-quality health care and prevention solutions closer to the Vietnamese people. At Takeda, we appreciate the strength of cooperation between medical partners in each country to work together to solve public health challenges, including solutions in preventing dengue fever".
After the signing ceremony, Takeda also coordinated with the Pasteur Institute of Ho Chi Minh City to organize a workshop on "Orientations and solutions to improve the effectiveness of dengue fever prevention and control in Vietnam". This is an important event in a series of activities taking place within the framework of the 50th anniversary of the establishment of diplomatic relations between Vietnam and Japan.
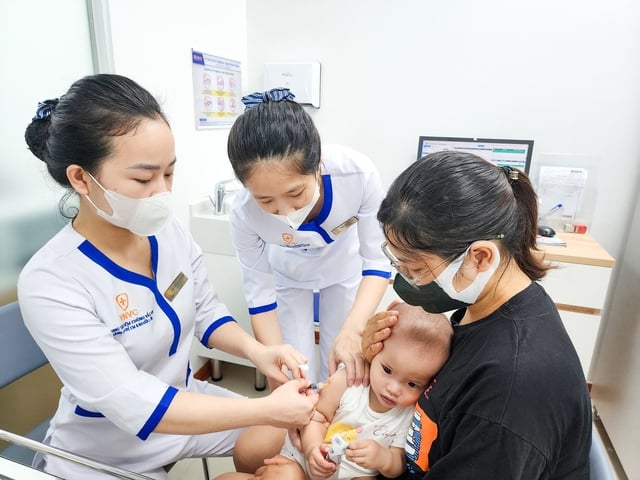
Children get vaccinated at VNVC Vaccination System
It is known that Takeda's dengue vaccine, TAK-003 (registered trade name QDENGA) has been licensed for use in more than 30 countries including the European Union (EU), the United Kingdom, Argentina and countries with similar dengue epidemics to Vietnam such as Indonesia, Brazil and more recently Thailand. TAK-003 is not currently licensed for circulation in Vietnam.
Test results showed that the vaccine can create immune responses at different levels against all four strains of dengue virus circulating in the world, helping to prevent disease and reduce the possibility of hospitalization in people with dengue fever.
According to the European Medicines Agency (EMA), the agency that licenses vaccines in the EU, the QDENGA vaccine has been approved for use in people aged 4 years and older, regardless of whether they have been previously infected or not.
With more than 130 centers nationwide, VNVC is the leading vaccination center system for children and adults in Vietnam. VNVC has a system of 4 cold storage centers and more than 130 cold storages meeting international GSP standards and cold chains at all vaccination centers nationwide, capable of storing up to 300 million doses of vaccine at the same time.
In addition to Takeda, VNVC is also a comprehensive strategic partner with many major vaccine manufacturers in the world such as GSK (Belgium), Sanofi (France), AstraZeneca (UK), Pfizer (USA), MSD (USA)...
Source link





![[Photo] National Assembly Chairman Tran Thanh Man meets with Thai Prime Minister Paetongtarn Shinawatra](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/15/e71160b1572a457395f2816d84a18b45)

![[Photo] Prime Minister Pham Minh Chinh receives Country Director of the World Bank Regional Office for Vietnam, Laos, Cambodia](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/15/2c7898852fa74a67a7d39e601e287d48)

























































































Comment (0)