Currently, clinical trials in Vietnam are mostly trials serving the development of new products such as: drugs, vaccines, medical equipment, new treatment techniques... In particular, in recent years there have been studies on cell and gene therapy.
Clinical trials need to be approved and closely evaluated before implementation because the testing process, new products and techniques can pose risks to volunteers participating in the research.
However, in reality, over the years there have been studies approved by ministries and sectors other than the Ministry of Health to conduct research on humans but not approved by the Council of Ethics in Biomedical Research.
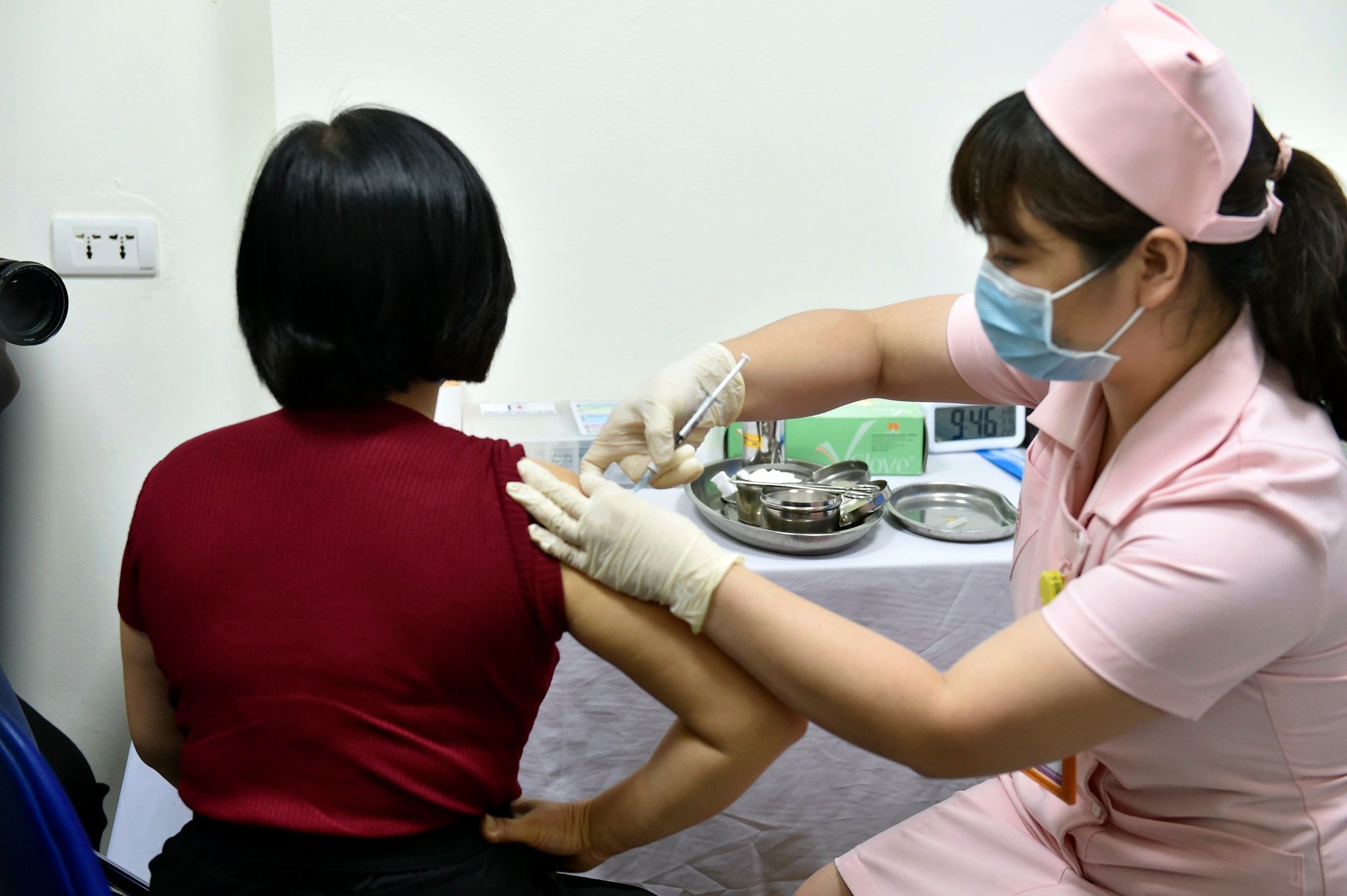
Experimental injection of Covid-19 vaccine Covivac at Hanoi Medical University, March 2021
The Ministry of Health is seeking comments on a draft circular regulating the management of scientific and technological tasks under the responsibility of the Ministry of Health. The draft proposes that research topics funded by non-budgetary sources, managed by other ministries and sectors but involving human research can only be carried out after approval by the Biomedical Research Ethics Council and approved by the Ministry of Health. At the same time, the draft also emphasizes and promotes the role of grassroots councils at units in approving research outlines, shortening approval time at the Ministry of Health, ensuring research progress.
In Vietnam, there are about 100 clinical trials every year, which is a favorable factor for the country to access new technologies, products, and methods in treatment and health care, especially for difficult diseases such as cancer, cardiovascular disease, etc. When new products and techniques are approved, Vietnam will also be the place to access them early, including drugs, vaccines, etc. However, before being officially licensed, the clinical trial process may pose risks to the health and life of volunteers, so it is necessary to be strictly approved and monitored during implementation.
According to current regulations, the Ministry of Health is responsible for managing, guiding, and organizing the implementation of biomedical research on human subjects. Scientific and technological tasks related to biomedical research on human subjects, before implementing, the organization in charge of the task must have the approval of the Ethics Council in biomedical research (according to the provisions of Circular No. 04/2020/TT-BYT).
Source link


![[Photo] Prime Minister Pham Minh Chinh holds talks with Prime Minister of the Kingdom of Thailand Paetongtarn Shinawatra](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/16/23b5dd1e595d429491a54e3c1548fb79)




![[Photo] Welcoming ceremony for Prime Minister of the Kingdom of Thailand Paetongtarn Shinawatra on official visit to Vietnam](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/16/cdd9e93739c54bb2858d76c3b203b437)






















































































Comment (0)