The Ministry of Health warns about fake Cefixim 200 antibiotics appearing on the market and requests that authorities recall and handle violating products, if any.
The Drug Administration of Vietnam, Ministry of Health has just sent a document to the Department of Health of provinces and cities regarding the counterfeit antibiotic Cefixim 200, in which it requested to coordinate with investigating agencies, verify information and trace the origin of the counterfeit Cefixim 200 product, and strictly handle organizations and individuals who violate...
 |
| The Ministry of Health warns about fake Cefixim 200 antibiotics appearing on the market and requests that authorities recall and handle violating products, if any. |
Accordingly, the Drug Administration Department said it had received an official dispatch with a Test Certificate from the Thanh Hoa Provincial Testing Center reporting that the product sample had the following labeling information: Cefixim 200 film-coated tablets, GĐKLH number: VD-28887-18, batch number: 15030723, NSX: 030723, HD: 030725, place of manufacture: Cuu Long Pharmaceutical Joint Stock Company.
The drug sample was taken by Thanh Hoa Provincial Testing Center at Hung Thinh Pharmaceutical Joint Stock Company (address: 207 Hung Long sub-area, Nga Son town, Nga Son district, Thanh Hoa province).
The drug sample did not meet the quality requirements for the qualitative index of Cefixim according to the basic standards. Afterwards, the Drug Administration received an official dispatch from the Binh Duong Provincial Testing Center, sending along a Test Report on the product sample with the following labeling information: Cefixim 200 film-coated tablets, GĐKLH number: VD-28887-18, batch number: 28201123, NSX: 201123, HD: 201125, place of manufacture: Cuu Long Pharmaceutical Joint Stock Company.
The drug sample was taken by Binh Duong Provincial Testing Center at Thanh Duy pharmacy (address: 174, group 4, Lai Khe, Lai Hung, Bau Bang district, Binh Duong province). The drug sample did not meet the quality requirements for the qualitative index of Cefixim according to the basic standards.
Then, on July 23, 2024, Cuu Long Pharmaceutical Joint Stock Company also issued Official Dispatch No. 284/DCL reporting the production of batches of CEFIXIM 200 and the differences between the drug samples kept at the company and the drug samples collected by the Thanh Hoa Provincial Testing Center on the market.
Based on these results, the Drug Administration of Vietnam has sent a document to the Department of Health of provinces and centrally-run cities to strengthen the prevention of counterfeit drugs CEFIXIM 200.
Accordingly, the Drug Administration of Vietnam requires the Department of Health of provinces and cities to notify drug businesses and users of information about counterfeit drugs on the label: CEFIXIM 200 film-coated tablets, registration number: VD-28887-18; batch number: 15030723, manufacturing date: 030723, manufacturing date: 030725, batch number: 04200623, manufacturing date: 200623, manufacturing date: 200625 and batch number: 28201123, manufacturing date: 201123, manufacturing date: 201125; Manufacturing facility: Cuu Long Pharmaceutical Joint Stock Company has the following characteristics and distinguishing signs from genuine drugs:
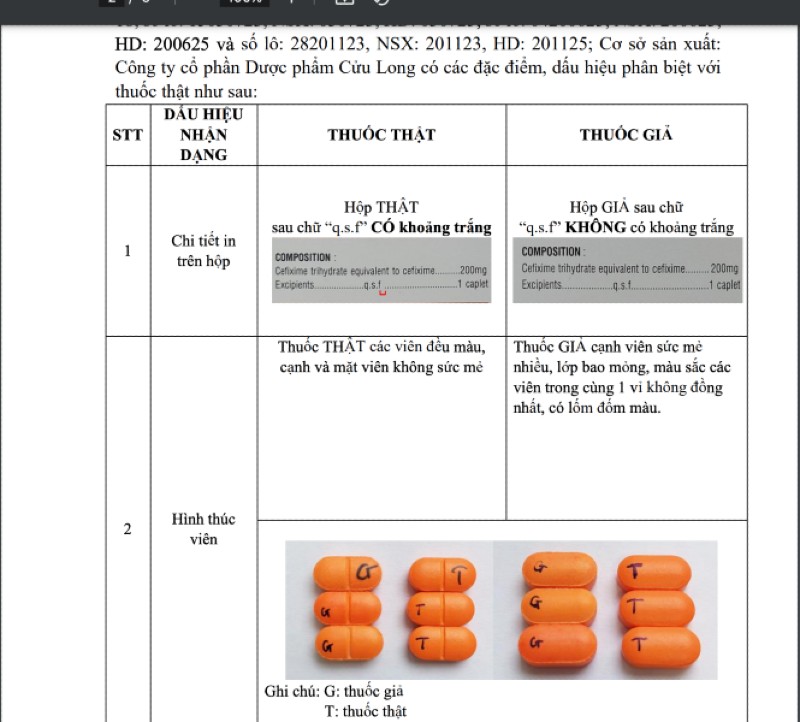 |
| Signs to distinguish real and fake drugs. |
Through the above incident, the Drug Administration of Vietnam requests relevant agencies to continue to strictly implement Directive 17/CT-TTg of the Prime Minister on strengthening the fight against smuggling, trade fraud, production and trading of counterfeit and poor quality goods in the group of pharmaceuticals, cosmetics, functional foods, medicinal herbs and traditional medicine.
At the same time, strictly implement the instructions in Official Dispatch No. 7173/BYT-QLD of the Ministry of Health on strengthening management, checking drug quality, fighting against counterfeit drugs and drugs of unknown origin.
Coordinate with media agencies to inform drug trading and usage establishments and people not to trade or use fake Cefixim 200 products with the above-mentioned identifying signs; only buy and sell drugs at legal pharmaceutical businesses;
Do not buy or sell drugs of unknown origin; promptly report any suspicious signs of production or trading of counterfeit drugs or drugs of unknown origin to health authorities and relevant authorities.
Coordinate with the police, market management agencies, Steering Committee 389 and relevant authorities to conduct inspections of drug trading establishments in the area;
Investigate, verify information and trace the origin of the above fake Cefixim 200 product, handle violating organizations and individuals according to current regulations;
At the same time, promptly detect and prevent the production, trade and use of fake Cefixim 200. Direct the testing center to increase sampling and quality control of drugs circulating in the area for drugs at risk of being counterfeited or of poor quality; promptly report detected cases to the Department of Health and relevant authorities.
Set up a hotline to receive information about counterfeit drugs, smuggled drugs, and drugs of unknown origin in the area to promptly coordinate with authorities to verify and handle.
Also regarding the issue of counterfeit drugs, recently, the authorities also received documents from the Representative Office of F. Hoffmann-La Roche Ltd. Switzerland in Hanoi informing about the discovery of a product sample suspected to be counterfeit, with the label stating: Actemra 400 mg/20 mL, batch number B2101B32.
The sample of the suspected counterfeit drug provided by the customer was compared, cross-checked and confirmed by F. Hoffmann-La Roche Ltd. to have different signs compared to the batch sample of Actemra 400 mg/20 mL, batch number B2101B32 distributed by this company and circulated only in Türkiye.
According to information from the Vietnam Pharmaceutical Business Association, most of the counterfeited pharmaceutical samples are antibiotics, often expensive antibiotics from famous pharmaceutical brands.
Counterfeit drugs are produced quite sophisticatedly, the only difference can be detected when comparing the packaging and instructions for use of real and fake drugs placed side by side.
The representative of the market management agency also admitted that, by conventional methods, it is not easy to accurately detect real and fake.
Testing the ingredients and quality of drugs is the optimal method to determine the authenticity of a product, but it requires a large amount of money and a long time to evaluate and investigate. This is also a major barrier to detecting and promptly handling the problem of fake drugs and fake functional foods on the market.
Applying technology to identify and trace product origin
The rampant counterfeit drug problem poses a great danger to patients. When using counterfeit drugs, patients may have to spend a lot of money to buy the drugs, but the disease does not improve, but even increases, causing panic and fear for patients because they think they are in a state of "no cure".
Counterfeit drugs also make treatment difficult, because they can neutralize treatment therapies. In cases where a patient is seriously ill and needs to use special drugs or antibiotics, if they use counterfeit drugs, the golden time to save the patient's life will pass, leading to increasingly serious consequences, even death.
Patients taking counterfeit drugs can lead to the emergence of drug-resistant bacteria, forcing doctors to change treatment procedures, prolonging treatment time and increasing costs.
People with chronic diseases such as high blood pressure, diabetes, asthma, etc. (who must take medication regularly and for a long time) if they take counterfeit drugs, it is very dangerous for their lives, because the treatment process is ineffective and the disease becomes more and more severe.
In some cases, counterfeit drugs contain poor quality active ingredients and pharmaceuticals due to the manual production process, the manufacturer mixes in many other impurities, and may even contain toxic substances, which can lead to death for the user.
Doctor Nguyen Trung Nguyen, Director of the Poison Control Center (Bach Mai Hospital) recommends that people should buy medicine at a trusted address, absolutely do not buy "hand-carried" medicine, medicine sold on the Internet in countries that do not have laws to control this type of medicine sale.
Faced with the problem of counterfeit and fake drugs, Mr. Le Van Truyen, Chairman of the Advisory Council for Drug Registration, Ministry of Health, said that genuine enterprises and manufacturers need to invest in research and development of advanced and modern anti-counterfeiting technology to help consumers and managers easily identify brands and trace product origins. This is also an effective way to protect the image of manufacturers and their products, and prevent counterfeiting.
Mr. Vu Tuan Cuong, Director of the Department of Drug Administration, Ministry of Health, requested the Department of Health of provinces and cities to strengthen inspection and examination of drug trading, wholesale and retail establishments in the area, ensuring that drug trading has full invoices and valid documents; promptly detect and prevent trading of drugs of unknown origin and smuggled drugs.
At the same time, coordinate with media agencies to widely inform people not to buy medicine on the Internet, only buy medicine at legal pharmaceutical businesses, with full invoices and documents proving the origin and quality of the medicine.
Medicines are special products, related to human health. Therefore, it is necessary to resolutely eliminate fake, counterfeit and poor quality products to protect people's health. People should also be vigilant against fake and counterfeit medicines, especially with products of unknown origin.
Source: https://baodautu.vn/canh-bao-thuoc-khang-sinh-cefixim-200-gia-d221102.html


![[Photo] President Luong Cuong presents the 40-year Party membership badge to Chief of the Office of the President Le Khanh Hai](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/a22bc55dd7bf4a2ab7e3958d32282c15)
![[Photo] Panorama of the Opening Ceremony of the 43rd Nhan Dan Newspaper National Table Tennis Championship](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/5e22950340b941309280448198bcf1d9)

![[Photo] Close-up of Tang Long Bridge, Thu Duc City after repairing rutting](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/086736d9d11f43198f5bd8d78df9bd41)

![[Photo] General Secretary To Lam attends the conference to review 10 years of implementing Directive No. 05 of the Politburo and evaluate the results of implementing Regulation No. 09 of the Central Public Security Party Committee.](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/2f44458c655a4403acd7929dbbfa5039)


























![[Photo] Prime Minister Pham Minh Chinh inspects the progress of the National Exhibition and Fair Center project](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/19/35189ac8807140d897ad2b7d2583fbae)



























































![[VIDEO] - Enhancing the value of Quang Nam OCOP products through trade connections](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/17/5be5b5fff1f14914986fad159097a677)





Comment (0)