The Drug Administration of Vietnam, Ministry of Health has just announced decisions to grant circulation registration certificates and extend circulation registration certificates for 355 domestically produced and imported drugs.
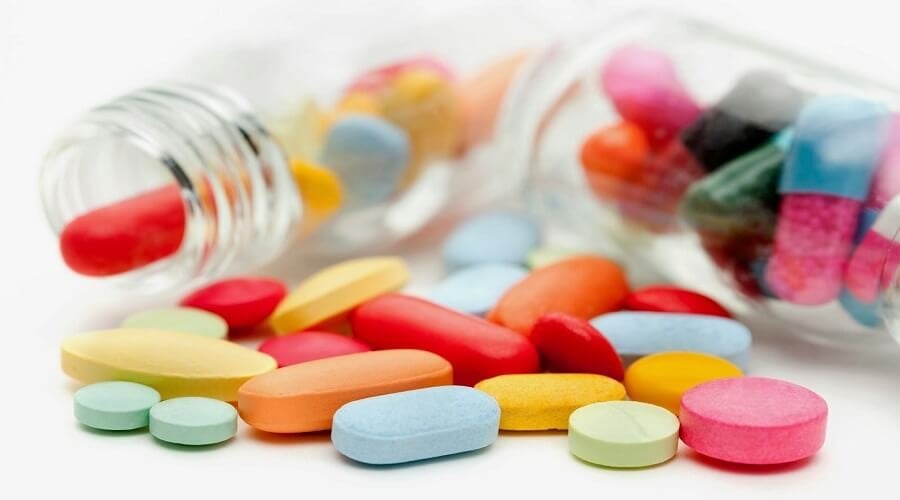 |
The Ministry of Health has just issued new and renewed registration certificates for 355 types of drugs. (Illustration photo) |
Director of the Department of Drug Administration - Ministry of Health, Dr. Vu Tuan Cuong has consecutively signed 4 decisions to announce the extension and re-issuance of circulation registration certificates for domestically and internationally produced drugs. Accordingly, 355 types of drugs have their circulation registration certificates extended and re-issued, of which some are newly issued and extended for 3 years, some for 5 years depending on specific conditions.
The drugs that have been renewed and re-issued this time are quite diverse in terms of pharmacological effects, including drugs for treating cancer, cardiovascular disease, diabetes, antiviral drugs as well as other common antipyretic, analgesic, and anti-inflammatory drugs...
The Drug Administration of Vietnam requires that drug manufacturing and registration establishments are responsible for manufacturing drugs in accordance with the records and documents registered with the Ministry of Health and must print the registration number issued by the Vietnamese Ministry of Health on the drug label.
Specially controlled drugs may only be produced and put into circulation when there is a Certificate of eligibility for pharmaceutical business. The scope of business of specially controlled drugs is consistent with the scope of operation of the facility, meeting the provisions of Clause 5, Article 143 of Decree No. 54/2017/ND-CP of the Government detailing a number of articles and measures to implement the Law on Pharmacy.
At the same time, update the quality standards of drugs according to the provisions of Circular No. 11/2018/TT-BYT of the Minister of Health regulating the quality of drugs and pharmaceutical ingredients, Circular No. 03/2020/TT-BYT of the Minister of Health amending and supplementing a number of articles of Circular 11/2018/TT-BYT regulating the quality of drugs and pharmaceutical ingredients.
Implement and coordinate with the importing facility to comply with the provisions of Official Dispatch No. 5853/QLD-CL dated April 19, 2019 of the Department of Drug Administration on quality inspection of raw materials for making sartan drugs for drugs in the list in Article 1 issued with this Decision containing pharmaceutical ingredients in the sartan group.
Update drug labels and drug instructions according to the provisions of Circular No. 01/2018/TT-BYT of the Minister of Health regulating the labeling of drugs, drug ingredients and drug instructions within 06 months from the date of signing and promulgating this Decision, in the form of changing and supplementing the drug circulation registration certificate prescribed in Circular No. 08/2022/TT-BYT.
In addition, manufacturing and trading establishments must coordinate with treatment facilities to comply with current regulations on prescription drugs, monitor the safety, effectiveness, and unwanted effects of drugs on Vietnamese people, and synthesize and report according to regulations...
The drug registration facility must ensure that the operating conditions are maintained during the validity period of the drug and drug ingredient registration certificate. In case the operating conditions are no longer met, the registration facility must be responsible for changing the registration facility according to the provisions of Circular No. 08/2022/TT-BYT within 30 days from the date the registration facility no longer meets the operating conditions.
Previously, the Drug Administration of Vietnam has repeatedly issued new and extended circulation registration certificates valid for 3 or 5 years according to the provisions of the 2016 Pharmacy Law for many pharmaceutical products to serve the people's needs for medical examination and treatment and disease prevention.
According to the Ministry of Health, there are currently about 22,000 drug registration numbers with circulation visas with about 800 active ingredients of various types.
Source


![[Photo] General Secretary To Lam meets and expresses gratitude to Vietnam's Belarusian friends](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/11/c515ee2054c54a87aa8a7cb520f2fa6e)


![[Photo] General Secretary To Lam concludes visit to Russia, departs for Belarus](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/11/0acf1081a95e4b1d9886c67fdafd95ed)

![[Photo] General Secretary To Lam arrives in Minsk, begins state visit to Belarus](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/11/76602f587468437f8b5b7104495f444d)
























![[Photo] National Assembly Chairman Tran Thanh Man attends the Party Congress of the Committee for Culture and Social Affairs](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/11/f5ed02beb9404bca998a08b34ef255a6)






























































Comment (0)