
Mr. Ngo Chi Dung, Chairman of the Board of Directors and General Director of VNVC Vaccination System and Mr. Stephane Liceras, International Business Director of Sanofi Pharmaceutical Group, signed an agreement on cooperation in producing some of Sanofi's vaccines at the VNVC factory in Vietnam - Photo: Moc Tra
Proactively providing vaccines for the country
The signing ceremony took place right after the official state visit to France by General Secretary and President To Lam, with strong orientations on enhancing cooperation between the two countries within the framework of scientific cooperation and innovation in trade and investment. In particular, the health sector has a tradition of good cooperation between Vietnam and France.
In this cooperation, Sanofi will share its extensive knowledge and expertise in biotechnology with VNVC to facilitate the gradual production of essential vaccines at VNVC's factory in Vietnam.
This important cooperation not only increases easier access to vaccines for the Vietnamese people but also contributes to ensuring a stable and proactive vaccine supply and significantly reducing many costs.
Present at the signing ceremony, Associate Professor, Dr. Nguyen Thi Kim Tien, former Minister of Health of Vietnam, honorary president of the Vietnam - France Friendship and Cooperation Association, vaccine expert, said this is a memorable event in the field of vaccine production in Vietnam.
This is the first time a Vietnamese private enterprise has signed a cooperation agreement on technology transfer with a world- leading corporation like Sanofi to produce important vaccines right in Vietnam.
According to Ms. Tien, the Law on Pharmacy, the Law on Investment and the Law on Taxation, which are about to be promulgated by the National Assembly, will create maximum conditions for the transfer of high technology and techniques, especially for pharmaceuticals and vaccines. This will help Vietnamese enterprises develop, while ensuring that Vietnamese people have access to high-tech pharmaceutical products and vaccines.

VNVC is the first vaccination unit in Vietnam to invest heavily in a cold storage system for preserving vaccines that meets GSP standards according to international standards, including 4 general warehouses and hundreds of on-site central warehouses - Photo: Moc Tra
Enhancing cooperation between Vietnam and France
Mr. Burak Pekmezci, country director of Sanofi Vietnam, said: “With many years of experience in vaccine production, Sanofi wishes to share knowledge and contribute to the development of the healthcare sector as well as improve public health in Vietnam.”
Mr. Dinh Toan Thang, Ambassador Extraordinary and Plenipotentiary of Vietnam to France, also highly appreciated the cooperation between VNVC and Sanofi as a continuation of the policy of raising the level of cooperation between Vietnam and France, specifically in the field of health, especially vaccine production cooperation between two large enterprises of the two countries. Health cooperation between the two countries has always been focused on by senior leaders, experts and policy makers of both sides from the past to the present.
Mr. Ngo Chi Dung, Chairman of the Board of Directors and General Director of VNVC Vaccination System, said that in the near future, VNVC will build a vaccine and biological product manufacturing plant with the world's leading modern technology according to EU GMP standards (good manufacturing practices according to European standards) in Long An province.
VNVC focuses on first cooperating with Sanofi to produce some important vaccines and biological drugs, thereby proactively supplying them to Vietnam.
“This signing ceremony will play an important role in promoting discussions and cooperation, focusing on knowledge sharing, production cooperation and technology transfer, based on mutually agreed terms, in line with the strategic goals of both VNVC and Sanofi,” Mr. Ngo Chi Dung shared.
Over the past 8 years, VNVC has made great efforts to negotiate and import large quantities of important vaccines and new-generation vaccines. However, this activity is completely dependent on manufacturers and transport units and requires a lot of procedures, time and costs. Not to mention, many other important vaccines are currently being allocated to epidemic countries or priority markets.
Therefore, efforts to participate in the production chains of vaccine companies are an important strategy to proactively produce vaccines, limit vaccine shortages, and help Vietnam be proactive in epidemic prevention strategies.
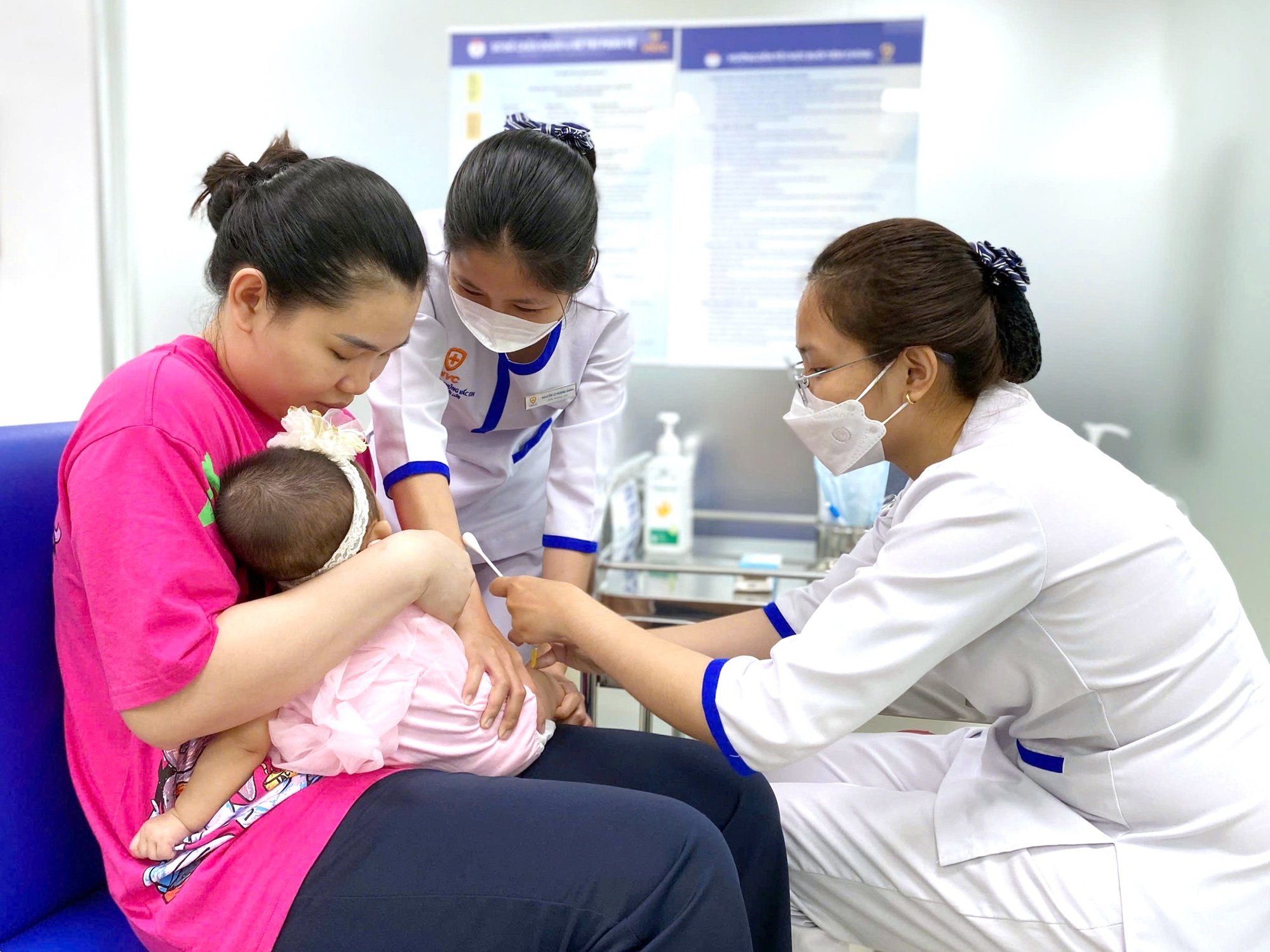
Children get vaccinated at VNVC - Photo: Moc Tra
How many types of Sanofi vaccines does VNVC have?
Currently, VNVC is deploying 10 types of Sanofi vaccines including: 6 in 1 Hexaxim, 5 in 1 Pentaxim, 4 in 1 Tetraxim, 3 in 1 diphtheria whooping cough tetanus Adacel, Japanese encephalitis Imojev, rabies Verorab, meningococcal meningitis Menactra, hepatitis A Avaxim, quadrivalent influenza Vaxigrip Tetra, typhoid Typhim VI.
According to the plan, VNVC will negotiate to gradually participate in the production and receive technology transfer of some vaccines in this portfolio of Sanofi.
Source: https://tuoitre.vn/vnvc-va-sanofi-ky-ket-tien-toi-hop-tac-san-xuat-vac-xin-tai-viet-nam-20241009161546502.htm





![[Photo] General Secretary To Lam receives First Deputy Secretary General of the African National Congress (ANC) of South Africa](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/20/bb2999907e1245d5b4c7310a890d8201)

































![[Photo] Vietnamese shipbuilding with the aspiration to reach out to the ocean](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/20/24ecf0ba837b4c2a8b73853b45e40aa7)
![[Photo] Award ceremony for works on studying and following President Ho Chi Minh](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/20/a08ce9374fa544c292cca22d4424e6c0)























































![[VIDEO] - Enhancing the value of Quang Nam OCOP products through trade connections](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/17/5be5b5fff1f14914986fad159097a677)
Comment (0)