The Drug Administration of Vietnam (Ministry of Health) has just issued Official Dispatch No. 113/QLD-CL warning about counterfeit drugs and drugs illegally circulating on the market to the Department of Health of provinces and centrally run cities.

The subjects producing fake drugs and the seized evidence. Photo: Thanh Hoa Provincial Police
Accordingly, on April 16, Thanh Hoa Provincial Police dismantled a large-scale nationwide counterfeit drug production and trading ring, arresting and prosecuting 14 defendants for the crime of "producing and trading in counterfeit disease prevention and treatment drugs".
Of the 21 products seized, four were identified as counterfeit drugs licensed for circulation by the Ministry of Health, including: Tetracycline, Clorocid, Pharcoter and Neo-Codion.
The remaining products do not match any drugs on the list that have been granted a circulation registration license by the Ministry of Health.
Pursuant to the provisions of the Law on Pharmacy, the Department of Drug Administration requests the Department of Health of provinces and cities and health sectors to urgently and widely notify drug businesses and facilities that they are not allowed to trade, sell, or use the following counterfeit products:
- Clorocid TW3 tablets (Cloramphenicol 250mg), registration number: VD-25305-16; manufacturer: TW3 Pharmaceutical Joint Stock Company, packaged in plastic bottles of 400 tablets.
-Tetracycline TW3 tablets (Tetracycline hydrochloride 250mg), registration number: VD-28109-17; manufacturer: TW 3 Pharmaceutical Joint Stock Company, packaged in plastic bottles of 400 tablets.
- Pharcoter tablets (Codein base 10mg; Terpin hydrate 100mg), registration number: VD-14429-11; manufacturer: TW1 Pharmaceutical Joint Stock Company (Pharbaco), packaged in plastic bottles of 400 tablets.
The Drug Administration of Vietnam said that the drug Neo-Codion, licensed by the Ministry of Health, has the following official information: license number 300111082223 (old registration number: VN-18966-15); active ingredient Codein base (in the form of Codein camphosulfonate 25mg) 14.93mg; Sulfogaiacol 100mg; Grindelia soft extract 20mg; dosage form is sugar-coated tablets; packaged in boxes of 2 blisters x 10 tablets. Manufacturer: Sophartex Company (France), address: 21, rue du Pressoir, Vernouillet, 28500.
There are 16 other counterfeit products (photo) that are not on the list of drugs that have been granted a circulation registration certificate by the Ministry of Health.
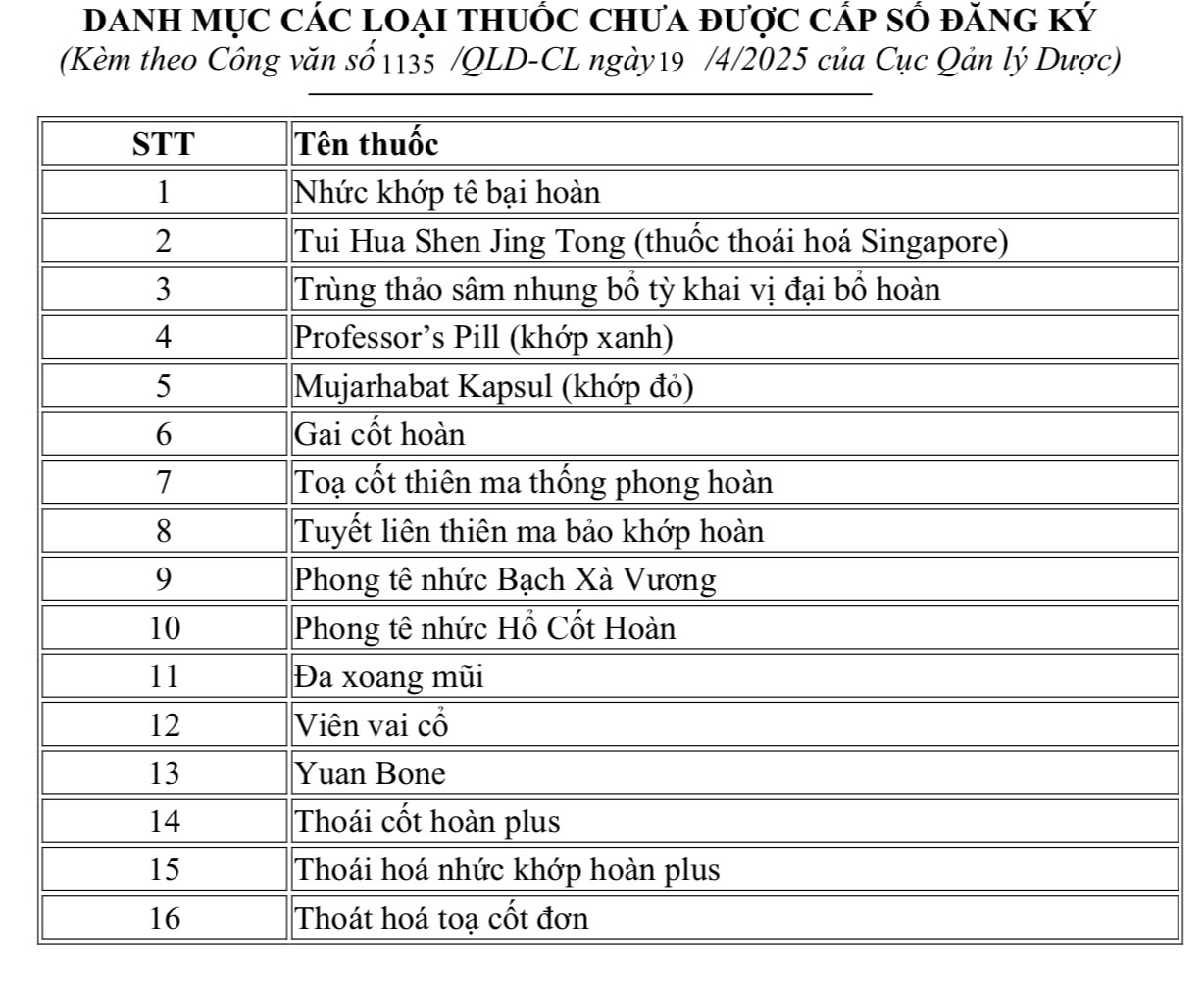
From this incident, the Drug Administration requested the Department of Health of provinces and cities to direct hospitals and medical examination and treatment facilities in the area to review the drug purchasing and supply process and the drug supply situation in the past; ensure that the drugs supplied are drugs that have been granted a circulation license and supplied by legal pharmaceutical businesses, with full invoices and documents.
In case of detecting any suspicious signs of abnormality in the drug or the drug has not been licensed for circulation, immediately seal it, do not continue to use the drug and report to the health management agency or competent authority for inspection, verification and handling according to the provisions of law.
“Set up a hotline to receive information about counterfeit drugs, smuggled drugs, and drugs of unknown origin in the area; promptly report to the local Steering Committee 389 and coordinate with relevant authorities to inspect drug trading establishments in the area, investigate, verify information and trace the origin of counterfeit drugs and drugs of unknown origin; strictly handle organizations and individuals who violate the law,” the Department of Drug Administration requested.
In addition, the Department of Health of provinces and cities directs functional units (Pharmaceutical Affairs Department/Pharmaceutical Practice Department...) to strengthen inspection and examination of pharmaceutical businesses, wholesale and retail establishments, and drug users in the area, focusing on checking the origin and source of drugs sold and used by businesses; maintaining compliance with the principles and standards of Good Distribution Practice (GDP), Good Retail Practice (GPP). On the other hand, directs the Testing Center to increase sampling and quality testing of drugs in circulation in the area for drugs at risk of being counterfeited or of poor quality.
Source: https://baotuyenquang.com.vn/sau-vu-21-loai-thuoc-gia-bo-y-te-yeu-cau-ra-soat-lai-quy-trinh-mua-thuoc-cung-ung-thuoc-210401.html



![[Photo] General Secretary To Lam arrives in Minsk, begins state visit to Belarus](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/11/76602f587468437f8b5b7104495f444d)
![[Photo] General Secretary To Lam meets and expresses gratitude to Vietnam's Belarusian friends](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/11/c515ee2054c54a87aa8a7cb520f2fa6e)

![[Photo] General Secretary To Lam concludes visit to Russia, departs for Belarus](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/11/0acf1081a95e4b1d9886c67fdafd95ed)

















![[Photo] National Assembly Chairman Tran Thanh Man attends the Party Congress of the Committee for Culture and Social Affairs](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/11/f5ed02beb9404bca998a08b34ef255a6)





























































Comment (0)