Ensuring access to medicines
On the morning of December 5, the Ministry of Health held a workshop to disseminate Circular 37 on the principles and criteria for developing, updating, recording information, structure of the list and payment instructions for pharmaceutical drugs, biological products, radioactive drugs and drug markers within the scope of benefits for health insurance participants.
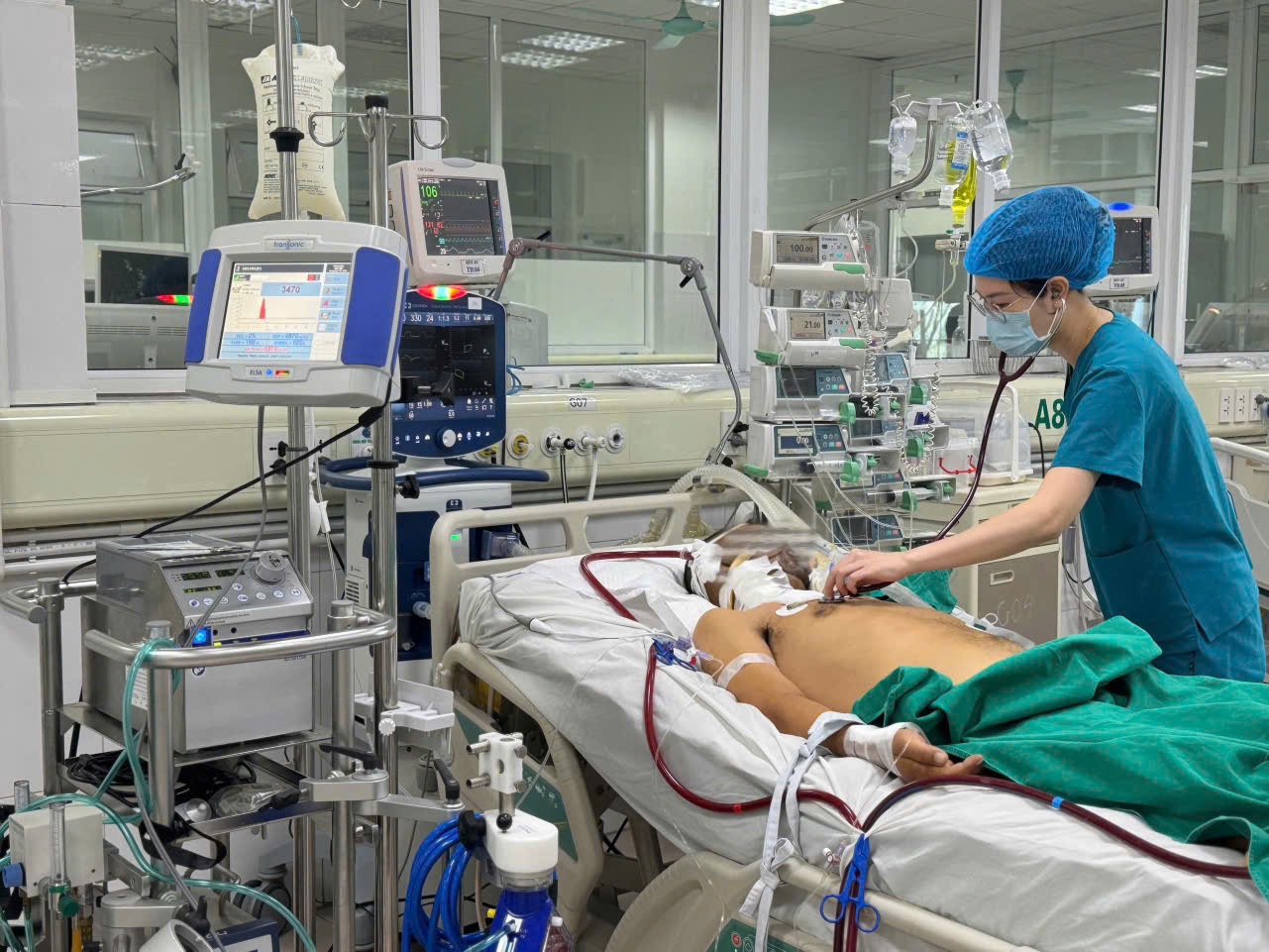
In the near future, many new drugs will be added to the list of health insurance drugs (illustrative photo).
Prof. Dr. Tran Van Thuan, Deputy Minister of Health, Chairman of the National Medical Council, said that medicine is always an important component, accounting for a large proportion of the total cost of medical examination and treatment under health insurance.
Currently, the issuance of the list and regulations on health insurance payment for drugs is being implemented according to Circular No. 20 of 2022. After nearly 2 years of implementation, Circular 20 has revealed a number of problems, requiring amendments, supplements, and adjustments to suit the actual situation.
On January 16, the Ministry of Health issued Circular No. 37 replacing Circular 20 with a number of new points. This Circular has added new regulations and amended a number of regulations on drug payment instructions to ensure compliance with the Law on Medical Examination and Treatment; Increase access to drugs, flexibility in payment instructions for patients; Create conditions for medical examination and treatment facilities to pay for drug costs that were not previously paid due to lack of specific instructions. Thereby, contributing to ensuring the rights of health insurance participants, while creating a financial mechanism to promote the development of medical examination and treatment facilities.
The representative of the Ministry of Health also emphasized that the regulations in this circular will encourage medical examination and treatment facilities to develop their expertise and techniques; attract human resources and encourage the development of the capacity of medical staff, especially creating conditions for the development of grassroots health care by ensuring fairness in access and payment of health insurance for drugs.
At the same time, it helps limit the number of patients choosing to go to medical examination and treatment facilities with high technical expertise, reducing the overload situation at some medical examination and treatment facilities with high technical expertise.
Proposal to add some new drugs
According to the leaders of the Ministry of Health, it is expected that in the first quarter of 2025, the Ministry of Health will develop and issue a circular on the list of drugs to replace Circular No. 20/2022/TT-BYT, focusing on reviewing and updating regulations on drug use according to hospital class and technical expertise level to match the technical expertise level as prescribed in the Law on Medical Examination and Treatment 2023.
Mr. Dong Huy Truong, Department of Health Insurance (Ministry of Health) said that in fact, this list has been slow to update recently, failing to meet the medical examination and treatment needs of health insurance participants in Vietnam. Many new and effective drugs have not been included in the list of health insurance drugs, so when prescribed, patients have to pay for themselves. This has increased the out-of-pocket payment rate of health insurance participants.
Therefore, the third principle when building and updating the drug list is to ensure the rights of health insurance participants, contributing to gradually reducing the direct payment rate of health insurance participants. In addition, depending on the payment capacity of the health insurance fund in each period, it is possible to adjust to narrow or expand the list, increase or decrease the payment rate...
According to Mr. Truong, the current list of pharmaceuticals, biologicals, radioactive drugs and markers covered by health insurance has 1,037 active ingredients. Up to this point, the Health Insurance Department has received 75 dossiers of drugs with proposed new additions with 25 groups of effects. Of these, the most are cancer treatment drugs, targeted therapy with high costs accounting for nearly 1/3.
Source: https://www.baogiaothong.vn/nhieu-thuoc-dieu-tri-ung-thu-duoc-de-xuat-vao-danh-muc-thuoc-bhyt-192241205143434578.htm


![[Photo] The moment Harry Kane lifted the Bundesliga trophy for the first time](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/11/68e4a433c079457b9e84dd4b9fa694fe)































































































Comment (0)