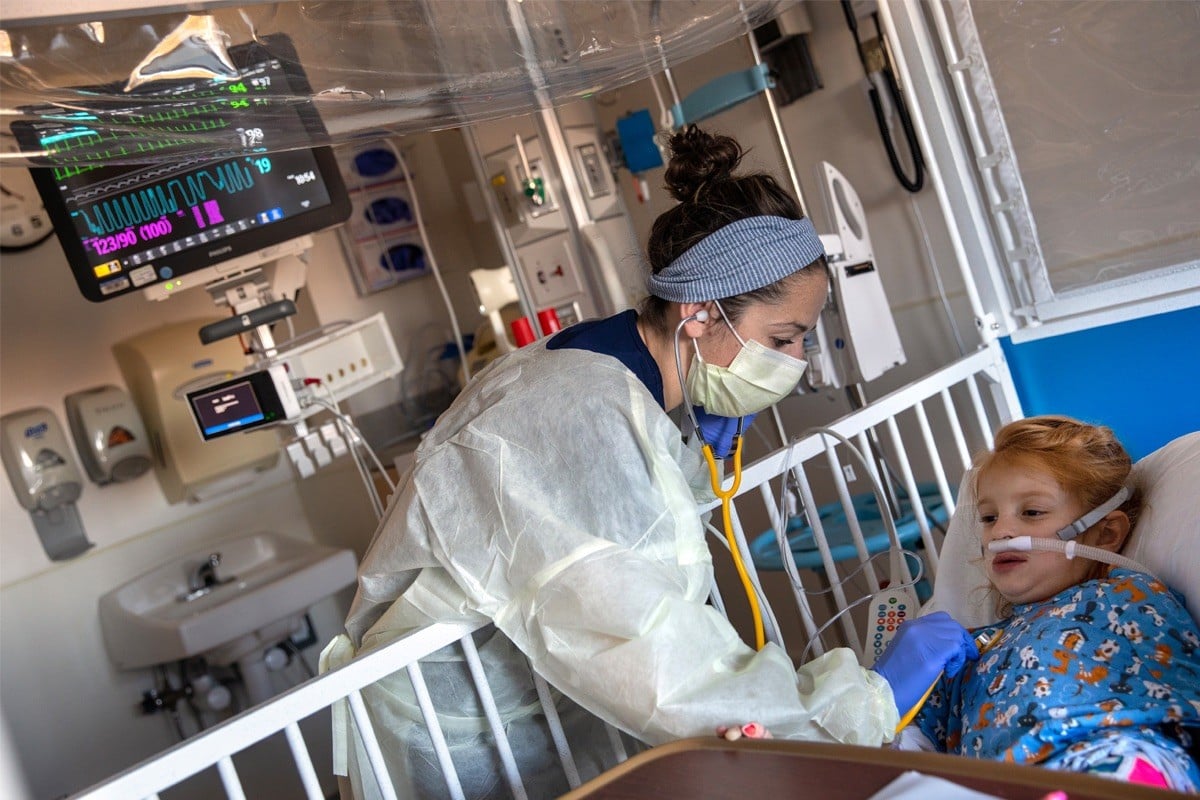
 |
| Respiratory syncytial virus (RSV) is one of the causes of serious respiratory illness in infants and young children. (Source: PennState Health News) |
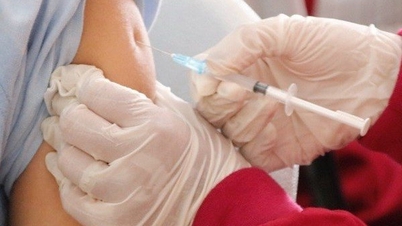
On May 18, an advisory panel of the US Food and Drug Administration (FDA) voted to recommend approving the first respiratory syncytial virus (RSV) vaccine for pregnant women to protect their newborns.
Accordingly, an independent advisory panel of experts voted to recommend giving this vaccine to pregnant mothers in the 24th-36th week of pregnancy. It takes several months for the FDA to review and grant approval.
NBC News reported that this vaccine, produced by Pfizer Pharmaceuticals, is the second RSV vaccine approved by the FDA in the US.
Earlier this May, the FDA approved the RSV vaccine produced by British company GlaxoSmithKline (GSK) for adults over 60. Currently, there are 11 RSV vaccines for different age groups in clinical trials.
According to the FDA, Pfizer's RSV vaccine data shows that it is safe for infants. Those who received the vaccine had a slightly higher rate of preterm birth than the other groups in the trial. However, this rate is still lower than the overall rate.
Among the 7,400 trial participants, the rate of premature birth was 5.7% in those who received the vaccine, 4.7% in those who received a placebo, and 10% in those who did not.
In a clinical trial, Pfizer's RSV vaccine reduced the risk of serious complications in the first three months of life by 82 percent in newborns and by 69 percent in babies older than 6 months, NBC News reported. Infants also had a 51 percent lower risk of respiratory illness severe enough to require medical attention.
RSV usually causes mild lower respiratory tract illness in healthy adults. However, people with weakened immune systems, such as the elderly and infants, are at high risk of infection. The virus can cause pneumonia or bronchiolitis, which can block the airways.
According to the US CDC, each year 58,000-80,000 children in the United States under the age of 5 are hospitalized for RSV and 100-300 children die.
Newborn babies' immune systems don't respond well to many vaccines, so it's difficult to give them directly. To protect newborns, Pfizer chose to vaccinate pregnant mothers a few months before giving birth, so their bodies would produce antibodies that would be passed on to their babies.
Side effects of the RSV vaccine may include fatigue, headache, muscle aches, and injection site pain in pregnant women after vaccination.
Source



![[Photo] VinUni students' emotions are sublimated with "Homeland in the Heart: The Concert Film"](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F26%2F1764174931822_10-3878-jpg.webp&w=3840&q=75)







































































































Comment (0)