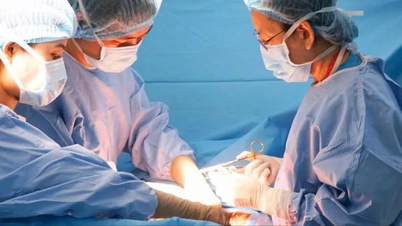
Sharing at the Workshop on Developing Circulars on Principles, Criteria for Developing, Updating, Recording Information, List Structure and Payment Instructions for Pharmaceutical Drugs, Biological Products, Radioactive Drugs and Markers within the scope of benefits of Health Insurance Participants (referred to as Circular on Payment Instructions for Pharmaceutical Drugs) held on October 25, Deputy Minister of Health Tran Van Thuan said that although in recent years the ratio of drugs/total health insurance examination and treatment expenses has continuously decreased, it still accounts for the largest proportion of expenditures from the Health Insurance Fund.
Specifically, in 2020 it was 40.42 trillion VND, accounting for 34.75%; in 2021 it was 34.48 trillion VND, accounting for 34.86%; in 2022 it was 40.57 trillion VND, accounting for 33.41%.
Currently, payment of drug costs for health insurance participants is implemented according to the list and regulations in Circular No. 20/2022/TT-BYT dated December 31, 2022 of the Ministry of Health , effective from March 1, 2023.
Accordingly, the list of drugs covered by health insurance includes: 1,037 active ingredients/chemical drugs and biological products divided into 27 large groups and 59 radioactive drugs and markers.

However, Circular No. 20 has revealed a number of limitations and problems that need to be adjusted and revised to suit reality and is outdated compared to many other documents and regulations in the medical field.
“For example, regarding payment for drugs used in remote medical examination and treatment and support for remote medical examination and treatment, the Law on Medical Examination and Treatment 2023 has currently stipulated many contents related to remote medical examination and treatment and support for remote medical examination and treatment. However, the list of health insurance drugs does not yet have regulations on health insurance payment for drugs used in remote medical examination and treatment,” said the Deputy Minister of Health.
Ms. Tran Thi Trang, Director of the Department of Health Insurance (Ministry of Health) said that the drafting of the Circular guiding the payment of chemical drugs will update new drugs that have been granted circulation registration numbers in the past, bringing high treatment efficiency to be able to contribute to the diagnosis and treatment of diseases at all levels from central to grassroots health care.
In addition, review the entire current drug list to remove any drugs with warnings related to treatment, low treatment effectiveness, or drugs with no longer suitable cost effectiveness from the list.
Thus, there will be a balance between adding new drugs to the list and removing ineffective drugs from the list so that health insurance participants can enjoy the benefits of using drugs more effectively and reasonably.

Ms. Trang affirmed that this draft Circular also includes regulations to help lower-level medical examination and treatment facilities access the best and most effective treatment drugs, suitable for the professional capacity of the licensed facility. The drug list will be updated regularly, at least once a year.
“This time I have separated the list of drugs for the commune health station. This way, the medical staff at the lower level will know which drugs are allowed to be used, can be proactive in purchasing, have enough drugs to promptly supply drugs to patients, and treat diseases promptly,” said the Director of the Health Insurance Department.
On the same day, the Department of Health Insurance also organized a workshop to develop a Circular promulgating principles, criteria for developing, updating the list and payment instructions for herbal medicines, traditional medicines, medicines combining pharmaceutical ingredients with herbal medicines, herbal medicines, traditional medicines within the scope of benefits of health insurance participants.
Accordingly, the Health Insurance Fund will pay for the cost of finished drugs used for patients, including the actual cost of drugs used for patients and the cost of loss of those drugs (if any) at medical examination and treatment facilities.
Traditional medicinal herbs and medicines are considered for addition to the list of health insurance drugs when they are effective and safe.
According to the Department of Health Insurance, the principle of building the list of health insurance drugs in the circular is to meet the people's need for traditional medicine treatment, ensure the rights of health insurance participants and be consistent with the payment capacity of the Health Insurance Fund.
Source: https://cand.com.vn/y-te/de-xuat-thanh-toan-bao-hiem-y-te-doi-voi-thuoc-kham-chua-benh-tu-xa-i748290/


![[Photo] Visit Hung Yen to admire the "wooden masterpiece" pagoda in the heart of the Northern Delta](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F21%2F1763716446000_a1-bnd-8471-1769-jpg.webp&w=3840&q=75)

![[Photo] General Secretary To Lam receives President of the Senate of the Czech Republic Milos Vystrcil](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F21%2F1763723946294_ndo_br_1-8401-jpg.webp&w=3840&q=75)
![[Photo] National Assembly Chairman Tran Thanh Man holds talks with President of the Senate of the Czech Republic Milos Vystrcil](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F21%2F1763715853195_ndo_br_bnd-6440-jpg.webp&w=3840&q=75)

![[Photo] President Luong Cuong receives Speaker of the Korean National Assembly Woo Won Shik](/_next/image?url=https%3A%2F%2Fvphoto.vietnam.vn%2Fthumb%2F1200x675%2Fvietnam%2Fresource%2FIMAGE%2F2025%2F11%2F21%2F1763720046458_ndo_br_1-jpg.webp&w=3840&q=75)

























































































Comment (0)