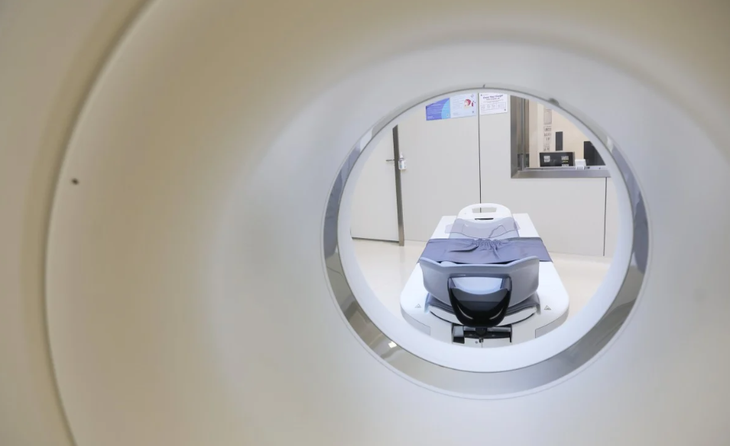
CT scan image at Shenzhen United Family Hospital on May 30, 2024 - Photo: SCMP
SCMP reported on April 20 that Damo Academy - the research arm of Alibaba Technology Group - has just received certification from the FDA for its cancer detection tool using artificial intelligence (AI).
The FDA has granted Damo Panda “breakthrough device” status, which expedites the review and approval process.
Alibaba first announced Damo Panda, a tool designed to detect pancreatic cancer, in January 2023 through an article published in the medical journal Nature Medicine .
According to information from Alibaba, Damo Panda can effectively detect malignant tumors at an early stage even in asymptomatic patients.
In theory, Damo Panda is a deep learning model (deep neural network) trained on 3,208 non-contrast computed tomography (CT) scans of the abdomen of pancreatic cancer patients.
According to researchers at Damo Academy, Damo Panda is 34.1% more sensitive than radiologists in detecting diseases.
Alibaba has deployed Damo Panda in trials across China. At a hospital in the city of Ningbo, the AI tool screened 40,000 people and detected six early-stage cancers, including two that were missed by conventional tests.
Alibaba said Damo Academy will continue to promote AI models at home and abroad by collaborating with companies such as Ankon Medical Technologies.
Amid the current tech boom, Alibaba is among the Chinese tech giants pouring resources into developing AI applications specifically for healthcare. Alibaba established Damo Academy in 2017, focusing on areas including AI and the open-source chip architecture RISC-V.
According to SCMP , not only Alibaba, telecommunications equipment giant Huawei is also focusing on the medical and healthcare industry when it launched an AI model in treatment at Ruijin Hospital, Shanghai, in March, with the aim of assisting doctors in diagnosing cancer.
Source: https://tuoitre.vn/alibaba-technology-recognized-by-fda-is-a-dot-pha device-2025042114240205.htm


![[Photo] Ho Chi Minh City residents stay up all night waiting to watch the parade rehearsal](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/27/0c555ae2078749f3825231e5b56b0a75)



![[Photo] Young people line up to receive the special supplement commemorating the 50th anniversary of the Liberation of the South of Nhan Dan Newspaper](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/26/9e7e624ae81643eba5f3cdc232cd07a5)
![[Photo] Spreading passion for science and technology in educational environment](https://vstatic.vietnam.vn/vietnam/resource/IMAGE/2025/4/27/059521b98e3847368f5ff4120460a500)
















































































Comment (0)