21 counterfeit drugs are not allowed to be traded or used.
Deputy Director of the Department of Drug Administration ( Ministry of Health ) Ta Manh Hung has just issued Official Dispatch No. 113/QLD-CL to health departments to notify 21 types of fake drugs in the case in which Thanh Hoa Provincial Police have dismantled a large-scale fake drug production and trading ring nationwide.
Of the 21 products seized, four were identified as counterfeit drugs licensed for circulation by the Ministry of Health , including: Tetracycline, Clorocid, Pharcoter and Neo-Codion.
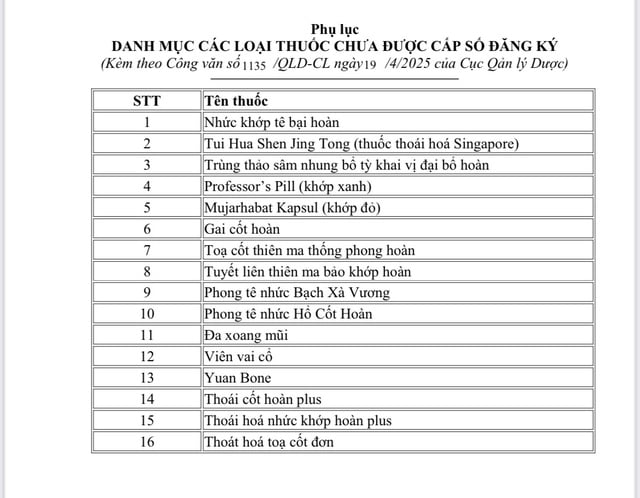
Of the 21 counterfeit drugs, 16 had labels that did not match those of the drugs that had been granted registration numbers.
PHOTO: DEPARTMENT OF DRUG ADMINISTRATION
The remaining products do not match any drugs on the list that have been granted a circulation registration license by the Ministry of Health.
Pursuant to the provisions of the Pharmacy Law, the Department of Drug Administration requests the provincial and municipal health departments and health sectors to urgently and widely notify drug businesses and facilities that they are not allowed to trade, sell, or use the following counterfeit products:
Clorocid TW3 tablets (Cloramphenicol 250mg), Registration number: VD-25305-16; Manufacturer: TW3 Pharmaceutical Joint Stock Company, packaged in plastic bottles of 400 tablets.
Tetracycline TW3 tablets (Tetracycline hydrochloride 250mg), registration number: VD-28109-17; manufacturer: TW 3 Pharmaceutical Joint Stock Company, packaged in plastic bottles of 400 tablets.
Pharcoter tablets (Codein base 10mg; Terpin hydrate 100mg), registration number: VD-14429-11; manufacturer: TW1 Pharmaceutical Joint Stock Company (Pharbaco), packaged in plastic bottles of 400 tablets.
Counterfeit drug product Neo-Codion.
The Drug Administration of Vietnam said that the drug Neo-Codion, licensed by the Ministry of Health, has the following official information: license number 300111082223 (old registration number: VN-18966-15); active ingredient Codein base (in the form of Codein camphosulfonate 25mg) 14.93mg; Sulfogaiacol 100mg; Grindelia soft extract 20mg; dosage form is sugar-coated tablets; packaged in boxes of 2 blisters x 10 tablets.
Manufacturer: Sophartex Company (France), address: 21, rue du Pressoir, Vernouillet, 28500.
There are 16 other counterfeit products (photo) that are not on the list of drugs that have been granted a circulation registration certificate by the Ministry of Health.
Source: https://thanhnien.vn/bo-y-te-cong-bo-21-loai-thuoc-gia-185250420170229962.htm




![[Photo] Prime Minister Pham Minh Chinh chairs conference to promote public investment growth momentum](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/20/7d1fac1aef9d4002a09ee8fa7e0fc5c5)























































































Comment (0)