NDO - With the majority of National Assembly deputies present voting in favor, the National Assembly officially passed the draft Law amending and supplementing a number of articles of the Law on Pharmacy. Accordingly, the law stipulates ensuring adequate and timely supply of quality drugs at reasonable prices for the people's disease prevention and treatment needs, in accordance with the disease structure and requirements of national defense, security, and disease prevention and control.
On the afternoon of November 21, continuing the 8th Session, under the direction of Vice Chairwoman of the National Assembly Nguyen Thi Thanh, the National Assembly carried out the voting process to pass the Law amending and supplementing a number of articles of the Law on Pharmacy.
Previously, before the National Assembly deputies voted to pass the draft law, the National Assembly listened to the Chairwoman of the National Assembly's Social Committee Nguyen Thuy Anh present a report on receiving, explaining and revising the draft Law amending and supplementing a number of articles of the Law on Pharmacy.
 |
Chairwoman of the National Assembly's Social Committee Nguyen Thuy Anh presented a report explaining, accepting and revising the draft Law amending and supplementing a number of articles of the Pharmacy Law. (Photo: THUY NGUYEN) |
Next, before the National Assembly delegates pressed the button to approve the entire law, the National Assembly voted to approve two contents.
Specifically: Firstly, regarding Clause 23, Article 1 on the rights and responsibilities of establishments organizing pharmacy chains and pharmacies in the pharmacy chain: The voting results showed that 427/434 delegates participating in the vote voted in favor (accounting for 89.14%), the National Assembly approved this content.
Second, Clause 30, Article 1 on: Competence, records, procedures, time for granting and extending the registration certificate for circulation of drugs and pharmaceutical ingredients, the voting results show that 413/419 National Assembly delegates participating in the vote voted in favor (86.22%), the National Assembly approved this content.
Immediately after the National Assembly voted to approve the above two contents, Vice Chairwoman of the National Assembly Nguyen Thi Thanh proposed that National Assembly deputies vote to approve the entire Law amending and supplementing a number of articles of the Law on Pharmacy.
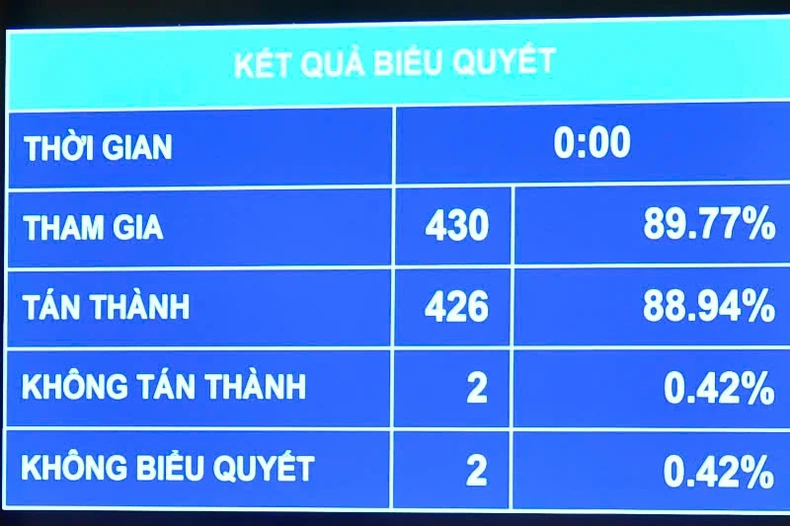 |
The National Assembly passed the Law amending and supplementing a number of articles of the Pharmacy Law with a high approval rate. (Photo: THUY NGUYEN) |
The voting results showed that 426/430 (88.94%) of the National Assembly delegates present agreed, and the National Assembly officially passed the Law amending and supplementing a number of articles of the Law on Pharmacy.
Accordingly, the Law amending and supplementing a number of articles of the Law on Pharmacy has clarified the concepts: Medicinal materials (including traditional medicinal ingredients), traditional medicine, traditional medicinal ingredients, biological products (also known as biological drugs)...
Regarding the State's policy on pharmaceuticals, the law stipulates: Ensuring adequate and timely supply of quality drugs at reasonable prices for the people's disease prevention and treatment needs, in accordance with the disease structure and national defense and security requirements, overcoming the consequences of incidents, natural disasters, catastrophes, and disease prevention and control. Ensuring rational, safe and effective use of drugs; having policies to develop clinical pharmacy and pharmacovigilance activities. Having preferential and supportive policies to develop the pharmaceutical industry into a spearhead industry.
 |
Delegates voting. (Photo: THUY NGUYEN) |
Regarding preferential policies and investment support in the development of the pharmaceutical industry, the law stipulates the implementation of preferential policies and investment support for investment projects in the pharmaceutical sector in accordance with the provisions of the law on investment.
New investment projects (including expansion of such newly established projects) in the development of the pharmaceutical industry with a total investment capital of VND 3,000 billion or more, disbursing at least VND 1,000 billion within 3 years from the date of being granted the Investment Registration Certificate or approved the investment policy, shall be entitled to special investment incentives and support as the subjects specified in Point a, Clause 2, Article 20 of the Investment Law.
Regarding the rights and responsibilities of the pharmacy chain organization and the pharmacies in the pharmacy chain, the law stipulates that the pharmacy chain organization has the following rights: To purchase pharmaceutical ingredients to transfer to pharmacies in the pharmacy chain to prepare prescription drugs and sell these drugs at the pharmacy that prepared them; to purchase drugs to transfer to pharmacies in the pharmacy chain for retail sale, except for vaccines...
Regarding drug price management measures, the law clearly stipulates: Bidding for national reserve drugs and bidding for drugs at medical facilities shall comply with the provisions of the law on bidding and the law on national reserves. Bidding, ordering or assigning tasks to supply drugs to serve national target programs, national defense, security, overcoming the consequences of incidents, natural disasters, catastrophes, disease prevention and control shall comply with the provisions of law...
The Law also stipulates recommendations on the expected wholesale price of drugs that have been announced or re-announced during the drug's circulation on the market when the Ministry of Health discovers one of the following cases: The expected wholesale price of a drug is higher than the highest price of a similar drug that has been announced or re-announced without a recommendation from the Ministry of Health; in case the drug has an expected wholesale price with a different content or concentration per unit dose than similar drugs, the price will be compared according to equivalent conversion; the difference between the expected wholesale price of a drug and the winning bid price of that drug is higher than the maximum difference prescribed by the Government...
Source: https://nhandan.vn/bao-dam-cung-ung-du-kip-thoi-thuoc-co-chat-luong-gia-hop-ly-cho-nhan-dan-post846146.html





![[Photo] Nearly 3,000 students moved by stories about soldiers](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/17/21da57c8241e42438b423eaa37215e0e)

![[Photo] Readers line up to visit the photo exhibition and receive a special publication commemorating the 135th birthday of President Ho Chi Minh at Nhan Dan Newspaper](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/5/17/85b3197fc6bd43e6a9ee4db15101005b)





















![[Photo] Readers line up to visit the photo exhibition and receive a special publication commemorating the 135th birthday of President Ho Chi Minh at Nhan Dan Newspaper](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/17/85b3197fc6bd43e6a9ee4db15101005b)

![[Photo] Nearly 3,000 students moved by stories about soldiers](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/5/17/21da57c8241e42438b423eaa37215e0e)



































































Comment (0)